Venous thromboembolism (VTE) in children is a rare disease usually as a secondary complication of primary underlying diseases such as congenital heart disease, sepsis, cancer, or after therapeutic interventions such as central venous lines (CVL; Table 1)[1,2].
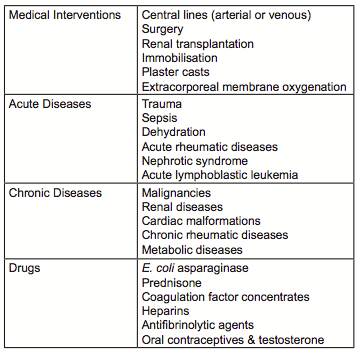
In children with cancer the rates of VTE vary on cancer type based on the diagnostic imaging modality and whether VTE was rated as symptomatic or asymptomatic. The rate of VTE events compared to pediatric controls is highest in children with hematologic malignancies, i.e. acute lymphoblastic leukemia (ALL) or lymphoma, followed by soft tissue sarcoma, bone tumors and malignancies of other sites[3].
The etiology of VTE in children with cancer is multifactorial and includes inherited risk factors (thrombophilia: IT), disease-related factors, and treatment-related factors including the use of CVL, the most common risk factor for VTE in children with cancer, surgery and/or chemotherapy[4]. A North-American multicenter case-control study of children with cancer recently identified age (≤2 and >10 years), blood group (non-O) and the use of E.coli asparaginase as independent risk factors for VTE onset[5]. In 2006 Caruso et al.[6] reported in children with ALL, that apart from the above mentioned risk factors[7,8] inherited thrombophilia (IT) contribute significantly to the risk of VTE.
Since based on intensified cancer treatment protocols the 5-year survival rates in children with cancer have been improved dramatically over the last four decades up to > 80%, treatment-related site effects, for example sepsis or VTE events, are increasingly diagnosed possibly leading to death or severe sequelae.
For example in children with ALL, approximately half of the VTE events occurred in the central nervous system resulting in long-term neurologic sequelae[9]. Further adverse outcomes of vascular accidents in children with cancer include the development of post-thrombotic syndrome (PTS) in approximately one third of VTE patients, associated with a history of CVL occlusion, VTE or multiple CVL insertions[10-12].
Treatment of thrombosis
Until results from randomized trials are available for anticoagulant treatment and duration of symptomatic index patients, children with clinically and imaging-proven VTE are treated according to recommendations based on small-scale studies in children and guidelines adapted from adult patient protocols[13].
Unfractionated heparin (UFH) , low-molecular weight heparins (LMWH), and vitamin K-antagonists are the commonly used antithrombotic drugs, whereas newly developed antithrombotic agents such as dabigatran, apixaban or rivaroxaban are under investigation in pediatric clinical trials.
Thrombosis prevention
Apart from adequate VTE treatment according to adult-adapted pediatric VTE guidelines evidence-based preventive strategies prior to the onset of VTE are sparse (PARKAA; 14): Very recently the THROMBOTECT study incorporating 949 patients gave evidence that children with ALL treated according to BFM protocols randomized to the LMWH enoxaparin or antithrombin replacement therapy developed less VTE events compared to children who received prophylactically UFH. The corresponding VTE rates were 3,5% (enoxaparin), 1,9% (antithrombin substitution) and 8,0% (UFH), respectively[15]. However, since s. c. administration of LMWH is still an additional heavy and painful burden for children with cancer, this option should be avoided if possible and restricted to high VTE risk patients only. Thus, such promising prophylactic strategies are available and should be administered if needed.
Children at risk could be identified by using risk assessment models such as proposed by Mitchell et al. in ALL[16]. This model may be adapted to local patient populations and treatment practice to prevent VTE. The latter model includes thrombophilia screening as one scoring point along with the use of CVL and prednisolone 10 mg/m2. Prior to discuss the necessity of any IT screening, however, we suggest investigating, if a positive VTE family history in pediatric cancer patients is known. If the family history with respect to VTE, early myocardial infarction or stroke is positive, an IT screening should be discussed, including severe IT, such as antithrombin-, protein C- or protein S deficiency, and factor V Leiden, since the latter is commonly associated with VTE during pediatric ALL treatment [BFM-adapted protocols: 16,17].
Such a step-wise VTE risk assessment including a selected individual IT screening procedure will allow antithrombotic/preventive treatment modifications: children at high risk only, identified via risk predicting models, could then be offered an antithrombotic preventive prophylaxis as suggested by Greiner et al.. Vice versa, children not at high VTE risk could for example avoid a painful s. c. LMWH prophylaxis, reducing an unnecessary bleeding.
In conclusion, taken together the possibility to modify primary VTE prevention in children with ALL based not only on
- disease-related and
- treatment related risk factors but also on
- genetic risk factors, here thrombophilia, we vote for an individual IT screening in selected pediatric cancer patient populations.
If, however, IT screening will modify antithrombotic treatment including dosage or duration in pediatric cancer patients compared to non-cancer patients will require further multicenter studies.
References
- van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. J Pediatr (2001) 139:676–81.10.1067/mpd.2001.118192
- Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics (2009) 124:1001–8.10.1542/peds.2009-0768
- Walker AJ, Grainge MJ, Card TR, West J, Ranta S, Ludvigsson JF. Venous thromboembolism in children with cancer – a population-based cohort study. Thromb Res (2014) 133:340–4.10.1016/j.thromres.2013.12.021
- Athale U, Siciliano S, Thabane L, Pai N, Cox S, Lathia A, et al. Epidemiology and clinical risk factors predisposing to thromboembolism in children with cancer. Pediatr Blood Cancer (2008) 51:792–7.10.1002/pbc.21734
- Spavor M, Halton J, Dietrich K, Israels S, Shereck E, Yong J, et al. Age at cancer diagnosis, non-O blood group and asparaginase therapy are independently associated with deep venous thrombosis in pediatric oncology patients: a risk model. Thromb Res (2016) 144:27–31.10.1016/j.thromres.2016.05.015
- Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, de Gaetano G, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood (2006) 108:2216–22.10.1182/blood-2006-04-015511
- Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br J Haematol (2007) 138:430–45.10.1111/j.1365-2141.2007.06677.
- Santoro N, Colombini A, Silvestri D, Grassi M, Giordano P, Parasole R,Barisone E, Caruso R, Conter V, Valsecchi MG, Masera G, Rizzari C. Screening for coagulopathy and identification of children with acute lymphoblastic leukemia at a higher risk of symptomatic venous thrombosis: an AIEOP experience. J Pediatr Hematol Oncol. 2013 Jul;35(5):348-55.
- Ranta S, Tuckuviene R, Makipernaa A, Albertsen BK, Frisk T, Tedgard U, et al. Cerebral sinus venous thromboses in children with acute lymphoblastic leukaemia – a multicentre study from the Nordic Society of Paediatric Haematology and Oncology. Br J Haematol (2015) 168:547–52.10.1111/bjh.13162
- Halton J, Nagel K, Brandao LR, Silva M, Gibson P, Chan A, et al. Do children with central venous line (CVL) dysfunction have increased risk of symptomatic thromboembolism compared to those without CVL-dysfunction, while on cancer therapy? BMC Cancer (2012) 12:314.10.1186/1471-2407-12-314
- Kuhle S, Spavor M, Massicotte P, Halton J, Cherrick I, Dix D, et al. Prevalence of post-thrombotic syndrome following asymptomatic thrombosis in survivors of acute lymphoblastic leukemia. J Thromb Haemost (2008) 6:589–94.10.1111/j.1538-7836.2008.02901.x
- Polen E, Weintraub M, Stoffer C, Jaffe DH, Burger A, Revel-Vilk S. Post-thrombotic syndrome after central venous catheter removal in childhood cancer survivors: a prospective cohort study. Pediatr Blood Cancer (2014) 62(2):285–90.10.1002/pbc.25302
- Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest (2012) 141:737S–801S.10.1378/chest.11-2308
- Mitchell LG, Andrew M, Hanna K, Abshire T, Halton J, Anderson R, et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with l-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer (2003) 97:508–16.10.1002/cncr.11042
- Greiner J, Schrappe M, Claviez A, Zimmermann M, Niemeyer C, Kolb R, et al. THROMBOTECT – a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica. 2018 Sep 27. pii: haematol.2018.194175.
- Mitchell L, Lambers M, Flege S, Kenet G, Li-Thiao-Te V, Holzhauer S, et al. Validation of a predictive model for identifying an increased risk for thromboembolism in children with acute lymphoblastic leukemia: results of a multicenter cohort study. Blood (2010) 115:4999–5004. doi:10.1182/ blood-2010-01-263012
- Sivaslioglu S, Gursel T, Kocak U, Kaya Z. The risk factors for thrombosis in children with acute lymphoblastic leukemia. Clin Appl Thromb Hemost. 2014, Sep;20(6):651-3.
