The HYPERCAN (HYPERcoagulation in CANcer) study (registered at www.clinicaltrials.gov; NCT02622815) is an ongoing, prospective, Italian, multicenter study, which started in 2012, aimed to investigate the association between the hypercoagulable state and cancer [1].
The goal of the study is to highlight an easy-to-detect laboratory tool for wide application in clinical practice, in order to identify individuals who are at a higher risk for cancer development or cancer recurrence.
The rationale of the study is based on the evidence that the hypercoagulable state is strictly associated with cancer for several reasons. Cancer cells express a series of clot-promoting factors, which may induce generation of thrombin and fibrin formation, but also the activation platelets, leukocytes and endothelial cells, which are all involved in the onset and amplification of the clotting cascade. Moreover, different coagulation proteins have been demonstrated to have important functions in tumor progression and spreading.
Clinical evidence has made it clear that patients who experienced venous thromboembolism may already be presenting malignancies that unfortunately remain hidden. Reliable screening tool(s) that can identify patients who are at risk for cancer development will increase the quality of prevention.
The HYPERCAN study involves a large cohort of subjects who have been carefully characterized by means of anthropometric, lifestyle and biological parameters to avoid the possible confounding effects. Patients are then followed-up for at least 5 years.
Another strength of the study consists of its design. In fact, it is divided into two arms. The first arm (project A), which involves healthy blood donors as subjects, evaluates whether the presence of a hypercoagulable state may be predictive of cancer development.
The second arm (project B), which involves newly diagnosed cancer patients (e.g. non-small-cell lung cancer, gastric cancer, colorectal cancer, breast cancer), evaluates the level of different hemostatic biomarkers, which will be used to predict the outcome of cancer or the occurrence of venous thromboembolism.
A subgroup of patients with early-stage, surgically resected, high-risk breast cancer, which are included in the project B of HYPERCAN study, were then selected to evaluate the baseline hypercoagulable status before starting systemic chemotherapy and investigated on the capacity of plasma thrombotic biomarkers to predict disease recurrence. The results of this study have recently been published in Haematologica [2]. To note, this is the first prospective study with a large sample size to assess hemostatic activation and its association with recurrence risk in surgically resected breast cancer patients.
A group of baseline circulating markers (D-dimer, fibrinogen, prothrombin fragment 1+2 [F1+2], and FVIIa/antithrombin [FVIIa-AT] complex) analyzed in participants in the HYPERCAN study, were evaluated in a cohort of 701 high-risk breast cancer patients who were scheduled for systemic chemotherapy, to predict disease recurrence over a 4-year period of follow-up.
Interestingly, it was found to be a moderate, but still significant, hypercoagulable state (i.e. increase in fibrinogen, F1+2, and D-dimer levels) in these patients (Figure 1).
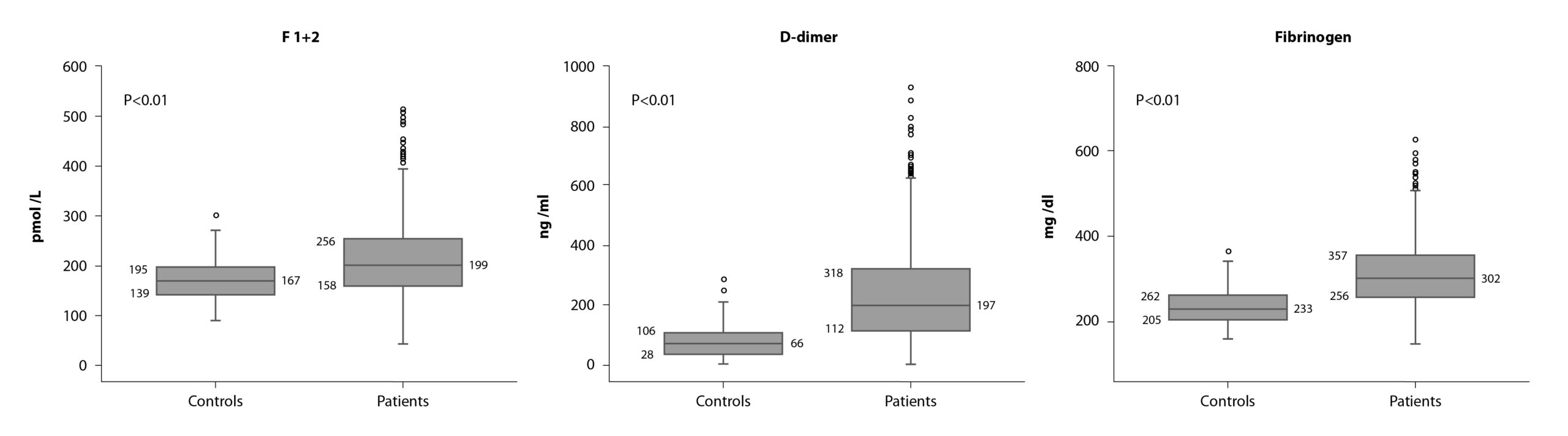
Correlations between the levels of each hemostatic markers with the time from surgery were performed showing no statistically significant correlation, therefore excluding a surgery-related effect and supporting, instead, a cancer-related effect.
The main and more intriguing finding was that levels of D-dimer and fibrinogen, positively and significantly correlated with tumor size and lymph node metastasis. In addition, pre-chemotherapy levels of fibrinogen were significantly and inversely associated with time to disease recurrence.
More importantly, patients who experienced recurrence showed significantly higher circulating levels of F1+2, compared with disease-free subjects; while high fibrinogen levels were significantly associated with a shorter time to disease recurrence. By cox-multivariate analysis, F1+2 was identified as independent risk factors for disease recurrence, together with tumor size, and luminal B HER2-negative or triple-negative molecular subtypes. Based on these variables, we generated a risk assessment model that significantly differentiated patients with higher risk (cumulative incidence: 6.2 vs 20.7%; hazer ratio = 3.5; p<0.001) of experiencing a recurrence after surgery.
This study is the first to demonstrate the utility of F1+2 as a potential circulating independent predictive biomarker for disease recurrence in a large cohort of patients with high-risk early breast cancer. F1+2 may potentially be helpful in identifying patients who need to be more accurately monitored.
These findings pave the way to further analyze independent cohorts of patients to confirm the results and stimulate careful monitoring of plasma markers of individuals subjected to surgery for primary breast cancer.
