Epidemiology and risk factors
Venous thromboembolism (VTE) is a burdensome and frequent complication in patients with malignancies, especially in those with the most advanced cancer disease[1]. Its development is associated with substantial morbidity and mortality[2], decreases quality of life[3], and can lead to interruption or discontinuation of cancer treatment. Although several studies have investigated the incidence of cancer-associated thrombosis (CAT) in ambulatory patients with malignancies[1], the exact rate has remained undefined until recently, as well as its association with potential risk factors for thrombosis.
In recent years, Decousus et al had the opportunity to follow-up prospectively for up to one year 3032 consecutive ambulatory patients with solid cancer, who had had the implantation of a port, mostly (97%) for infusion of chemotherapy (the ONCOCIP study)[4]. The median age was 63 years; 58% were women. The most frequent cancer locations were breast (34%), lung (19%) and colorectal (16%), cancer being metastatic in 43.2% of patients. Only 8.1% of patients were administered standard or low-molecular-weight heparin (LMWH) in prophylactic doses, and 15.1% antiplatelet drugs. By 12 months, overall thromboembolic complications occurred in 397 patients (13.8%; 95% CI: 12.6-15.0), including 111 with catheter-related thrombosis, 276 with symptomatic or incidentally detected VTE other than catheter-related, and 10 with arterial thromboembolism. Not surprisingly, in most patients the risk of thromboembolism extended far beyond the end of chemotherapy. Indeed, besides chemotherapy several factors are likely to account for the increasingly high rate of vascular complications occurring over time, including stage of disease, patient-related factors, specific conditions associated with cancer such as stasis due to immobilization, surgery or infections.
Hence, the incidence of overall thromboembolism in ambulatory cancer patients requiring the implantation of a port for the infusion of chemotherapy is remarkably high and surpasses by far that reported up to now. By multivariate analysis, use of the cephalic vein for catheter insertion predicted catheter-related thrombosis, whereas risk factors for VTE other than catheter-related were advanced age, previous VTE, cancer site and low hemoglobin level or increased leukocyte count before chemotherapy. Of interest, these risk factors largely reproduce those included in the Khorana score (see below). The main implication of the ONCOCIP study is that for a thromboprophylaxis to be effective in patients with advanced cancer there is the need for its administration for as long as the cancer is active.
Value and limits of stratification tools
The best-known risk stratification tool for the identification of cancer patients at the highest risk of chemotherapy-related CAT is the Khorana score, which was introduced in 2008[5]. This score assigns points to five clinical and pre-chemotherapy laboratory parameters [Table 1].

A sum score of 0 points classifies patients as being at low risk of VTE, 1 or 2 points at intermediate risk, and 3 or more points at high risk. Although this score is widely used, and is endorsed by the latest guideline updates of the ASCO and the NCCN to select ambulatory cancer patients for thromboprophylaxis[6,7], its value and limitations have been clarified only in January of this year, when the results of a comprehensive meta-analysis of 55 cohorts enrolling almost 35 000 cancer patients who had up to six months of follow-up were published[8].
Overall, 19% of patients had a Khorana score of 0 points, 64% had a score 1 or 2, and 17% had a score higher than 2 points. The incidence of VTE in the first 6 months was 5.1% (95% CI: 3.9-6.5) in the first group, 6.6% (95% CI: 5.6-7.7) in the second, and 11.0% (95% CI: 8.8-13.8) in the third, respectively. However, of the patients with VTE in the first 6 months, only 23.4% had been classified as being at a high risk according to the Khorana score. Of interest, this proportion increases up to 55% if the high-risk score is expanded to incorporate patients scoring 2, but the predictivity of CAT of a score > 2 decreases to 8.9%. Whether a 9% risk of VTE during the first 6 months is to be considered high enough to justify thromboprophylaxis is a matter of debate.
Based on these conclusions, the Khorana score in its original interpretation can be used to select ambulatory cancer patients at high risk of VTE for thromboprophylaxis, however, most events occur outside this high-risk group. Classifying patients as being at low (up to 1) or high risk (2 or more) is likely to provide a more useful tool to identify patients at risk of VTE, although more than 90% of patients at high risk will not develop chemotherapy-related VTE.
Recently, Pabinger et al have derived and validated (in two separate cohorts) a new interesting approach based on the combination of only two variables: the tumor site and the baseline D-dimer concentration (as expressed in ng/ml)[9]. To this purpose, cancer sites are classified as scoring 100 (pancreas and stomach), 50 (lung, colorectal, esophagus, kidney, lymphoma, bladder, uterus, cervical, ovarian, testicular, sarcomas), or 0 (breast, prostate). Points for D-dimer concentration and tumor-site risk category can be obtained by calibrating with the point caliper, and then combined to obtain a total score that can be calibrated with the cumulative 6-month incidence scale [Figure 1].
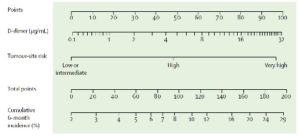
At a 10% cutoff for predicting the 6-month risk of VTE, the sensitivity is 21% (95% CI: 10–35), the specificity 87% (85–90), the positive predictive value 9% (4–16), and the negative predictive value 95% (93–96). Although this stratification algorithm is promising, there is the need for controlled studies addressing the value of thromboprophylaxis in patients identified as being at a high risk of CAT according to the Vienna model.
Prevention of VTE
Although thromboprophylaxis effectively reduces the risk of VTE[10], current guidelines recommend against its routine use in ambulatory cancer patients, due to the high number needed to treat, the fear of bleeding, and the considerable burden associated with daily injections of LMWH[6].
Recently, the value of direct oral anticoagulants (DOAC) for prevention of chemotherapy-related VTE has been investigated in two placebo-controlled double-blind randomized clinical trials addressing the value of apixaban (2.5 mg t.i.d) and rivaroxaban (10 mg o.i.d), respectively[11,12]. In both trials, cancer patients initiating chemotherapy were enrolled provided the Khorana score was 2 or higher, and were followed-up for up to six months.
In the AVERT study (addressing the value of apixaban), in the 6-month follow-up period VTE occurred in 12 of 288 patients (4.2%) in the apixaban group and in 28 of 275 patients (10.2%) in the placebo group (HR, 0.41; 95% CI: 0.26-0.65; P<0.001; NNT=17)[11]. Although the rate of major bleeding was higher in the apixaban group (3.5%) than in controls (1.8%), the difference disappeared when the analysis was confined to the treatment period (2.1 vs 1.1%; HR=1.89; 95% CI: 0.39-9.24).
In the CASSINI study (addressing the value of rivaroxaban), during the on-treatment period overall thromboembolism developed in 11 of 420 patients (2.6%) allocated to rivaroxaban, and in 27 of 421 (6.4%) randomized to placebo (HR=0.40; 95% CI 0.20-0.80; p=0.007; NNT=26) without any appreciable increase in the rate of major bleeding complications[12]. When the analysis was extended to cover the whole 6-month period (which was the intended primary end-point), the advantage of rivaroxaban did not achieve a statistically significant value. It should be considered, however, that over one-third of events occurred post discontinuation of study drug. Based on the inexorable risk of thromboembolism over the course of chemotherapy and beyond it in patients with active cancer, as shown by the ONCOCIP study[4], it is very unlikely for a drug to preserve its preventive value beyond the end of its administration.
Taken together, the results of these two clinical trials show that the administration of low-dose DOACs to ambulatory cancer patients who require chemotherapy, provide they do not present contraindications to the drugs and have a Khorana score of at least 2, has the potential to offer an effective and safe protection against thromboembolic events, in such a way obviating the burden and the cost of LMWH. Hopefully, the results of these two studies will be reputed strong enough to induce reconsideration of the current guidelines
References
- Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. Thromb Haemost 2016;117(1):57–65.
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5(3):632–4.
- Lloyd AJ, Dewilde S, Noble S, Reimer E, Lee AYY. What impact does venous thromboembolism and bleeding have on cancer patients’ quality of life? Value Heal 2018;21(4):449–55.
- Decousus H, Bourmaud A, Fournel P, et al. Cancer-associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood 2018;132(7):707-16.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Lancet 2008;111(10):4902–7.
- Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol 2015;33(6):654–6.
- Streiff MB, Holmstrom B, Ashrani A, et al. Cancer-associated venous thromboembolic disease, version 1.2015: Featured updates to the NCCN Guidelines. JNCCN J Natl Compr Cancer Netw 2015;13(9):1079–95.
- Mulder FI, Candeloro M, Kamphuisen PW, et al. The Khorana score for prediction of venous thromboembolism in cancer patients: a systematic review and meta-analysis. Haematologica 2019 [Epub ahead of print].
- Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol 2018;5(7):e289-98.
- Di Nisio M, Porreca E, Ferrante N, Otten HM, Cuccurullo F, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2012;2(12):CD008500.
- Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2018 [Epub ahead of print].
- Khorana AA, Soff GA, Kakkar AK, et al; CASSINI Investigators. Rivaroxaban thromboprophylaxis in high-risk ambulatory cancer patients receiving systemic therapy: results of a randomized clinical trial. Blood 2008 Blood 2018;132:LBA-1 [asbstract submitted to the ASH congress].
