Introduction
There is a clear relationship between malignancy and deranged coagulation[1]. Patients with cancer are at high risk to present with venous thromboembolism, which occurrence in some cases may precede the diagnosis of cancer. However, thrombotic complications of cancer are not restricted to thrombosis, and other manifestations of a prohemostatic state, in its most fulminant form presenting as disseminated intravascular coagulation (DIC), may occur as well [2,3]. The clinical manifestation of DIC in cancer may be thrombosis, bleeding,or a combination of these two conditions (Figure 1) [4]. In addition, thrombotic microangiopathy may occur [5].
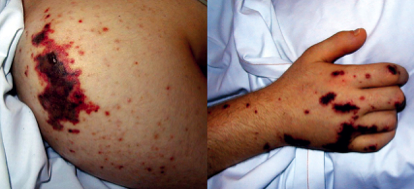
Thrombotic complications due to DIC in cancer
Mucin-secreting tumors, such as pancreas, lung, and stomach malignancy, or metastasized adenocarcinomas from unknown origin, are associated with the strongest coagulation activation and the highest risk of thrombosis [6]. Prospective studies have demonstrated that hypercoagulability is a particular risk in patients with brain tumors, ovarian cancer and pancreatic carcinoma [7,8]. In absolute terms, due to their relatively frequent prevalence, thrombotic complications in lung cancer, colon carcinoma and prostate tumors, are most frequently observed [9].
Chemotherapy may increase the thrombotic risk presumably due to its detrimental effect to endothelial cells. Anti-angiogenic drugs, especially when combined with chemotherapy, may significantly augment this risk. Administration of thalidomide in combination with chemotherapy for renal cell carcinoma or multiple myeloma caused very high complication rates of thrombosis in 43% and 28% of patients, respectively [10,11]. Other anti-angiogenic agents have been related to an enhanced risk of both venous and arterial thrombosis, likely caused by their effect on endothelial cells [12,13].
Bleeding in patients with cancer and DIC
Contrary to patients who present with fulminant DIC as a complication of sepsis or trauma, the coagulopathy in patients with cancer may manifest with relatively mild or insidious clinical symptoms of platelet and coagulation factor consumption or even non-symptomatic disease characterized by laboratory abnormalities only [14-16]. The clinical presentation of subacute to protracted forms of DIC usually occurs in association with mucin-producing adenocarcinomas and some types of acute hematological malignancies. The latter is often dominated by bleeding, whereas thromboembolic manifestations are more common in case of solid tumors.
In a series of 182 patients with cancer and DIC, major and profuse hemorrhage was seen in 75 cases, whereas thrombotic complications were more frequently present, in the form of venous thrombosis (123 cases), migratory thrombophlebitis (in 96 patients), and arterial thrombosis and embolism caused by nonbacterial thrombotic endocarditis (31 patients) [6]. In addition, multifocal hemorrhagic cerebral infarctions, caused by microemboli were described. A unique feature of patients with cancer and a prohemostatic state, in particular in those with mucin-producing carcinoma, is nonbacterial thrombotic endocarditis with systemic embolization [17].
Pathogenetic features of DIC in cancer
The pathways that contribute to activation of hemostasis and the ensuing prothrombotic state associated with malignant disease are for an important part understood (Figure 2). Tissue factor- and cancer-procoagulant-initiated activation of coagulation, cytokine-mediated dysfunctional anticoagulant systems and deranged fibrinolysis play a crucial role and pathological processes may evolve at the surface of endothelium injury caused by radio- and chemotherapy.
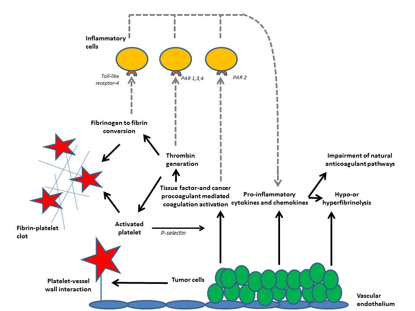
Binding of tissue factor/factor VIIa, thrombin, and other activated coagulation proteases to specific protease activated receptors (PAR’s) and binding of fibrin to Toll-like receptor 4 on inflammatory cells affects inflammation through the consequent release of pro-inflammatory cytokines and chemokines, which further modulate coagulation and fibrinolysis.
Malignant cells can express various procoagulant molecules such as tissue factor (TF), which binds circulating factor VII(a) and subsequently activates factors IX and X. Another initiator of cogulation is cancer procoagulant (CP), a cysteine protease with factor X activating properties [18]. An alternative mechanism by which tumor cells may contribute to the pathogenesis of DIC is by expressing fibrinolytic proteins. Despite the ability of many malignant cells to express plasminogen activators, such as urokinase-type plasminogen activator (u-PA) and tissue-type plasminogen activator (t-PA), which may cause a hyperfibrinolytic state [19]. An acquired deficiency of ADAMTS13 is sometimes seen in cancer, contributing to thrombotic microangiopathy that can complicate DIC [20].
The coagulopathy associated with acute promyelocytic leukemia, is typically seen as one of the most forthright variants of DIC complicating malignancy [21]. This form of leukemia-associated hemostatic derangement has, however, exceptional features manifested by a marked hyperfibrinolysis. The clinical picture of severe hemorrhage combined with laboratory findings of a marked hypofibrinogenemia, excessively high levels of fibrin degradation products, significant depletion of plasminogen and a2-antiplasmin lend support to that concept [22]. Some studies strongly suggest that the expression of a receptor for fibrinolytic proteins, annexin II on leukemic cells and facilitating plasmin generation may promote fibrinolysis in patients with acute promyelocytic leukemia.. Despite the prominent role of hyperfibrinolysis in patients with acute promyelocytic leukemia, there is sufficient evidence that it is superimposed on a more usual presentation of DIC, characterized by hemostatic activation and fibrin generation [23]. Indeed, diffuse thrombosis is found in 15-25% of acute promyelocytic leukemia patients at autopsy and recent studied have demonstrated tissue-factor dependent activation of coagulation in this condition [24].
Diagnosis and management of DIC in cancer
DIC in cancer has often a more gradual and sustained, systemic activation of coagulation which can initially proceed subclinically [25]. Eventually the ongoing activation of coagulation may result in exhaustion of platelets and clotting factors which can result in the occurrence of bleeding (typically at the site of the tumor). In some cases hemorrhage may be the first clinical symptom indicating the presence of DIC. As long as liver function is unaffected, increased synthesis of coagulation factor may disguise the ongoing consumption of clotting proteins and in most cases development of thrombocytopenia is the most significant sign of ongoing DIC. Measurement of fibrin-related markers (such as D-dimer or other fibrin degradation products) may be useful in making the diagnosis of DOC in most routine settings. however, the specificity of these tests for malignancy-related DIC is likely to be modest.
The foundation of the management of DIC is treatment of the underlying disorder. In fact, if the cancer can be brought in remission, DIC will typically spontaneously disappear [26,27]. Supportive therapy may consist of anticoagulant treatment, However, the efficacy and safety of this strategy in cancer patients with DIC has never been studied in sound clinical studies [28,29]. Treatment of overt thromboembolism by antithrombotic agents is obviously indicated, whereby low molecular weight heparin seems to be of advantage compared to other anticoagulant modalities [30,31]. Direct oral anticoagulants were shown to be slightly superior over vitamin K antagonists in this situation [32]. Experimental studies have demonstrated that heparin can at least partly block coagulation activation in DIC [33]. Despite the fact that thrombocytopenia and low concentrations of coagulation proteins may increase the risk of hemorrhage, plasma or platelet substitution therapy should not be instituted on the basis of laboratory results alone. This supportive treatment is only indicated in patients with active bleeding and in those requiring an invasive procedure or otherwise at risk for bleeding complications. The starting point for transfusion of platelets depends on the clinical condition of the patient. Typicallyplatelets are transfused to patients with active hemorrhage who have a platelet count of <50×109/l. In the absence of bleeding a much lower threshold for platelet transfusion is used (usually <10-20×109/l), which is inferred from randomized controlled trials in patients with thrombocytopenia following chemotherapy [34].In patients with severe hemorrhage, antifibrinolytic treatment may be contemplated [35]. However, since fibrin deposition in DIC may partly be due to inadequate fibrinolysis, further impairment of the fibrinolytic system may not always be appropriate and could cause thrombosis. An exception may be those cases in which primary or secondary hyperfibrinolysis governs the clinical picture, such as in acute promyelocytic leukemia (AML-M3) and in some cases of DIC secondary to adenocarcinomas. Uncontrolled observations and one randomized controlled clinical trial have demonstrated the effectiveness of antifibrinolytic agents in this setting [36].
References
- Levi M. Clinical characteristics of disseminated intravascular coagulation in patients with solid and hematological cancers. Thromb Res. 2018;164 Suppl 1:S77-s81.
- Levi M. Cancer-related coagulopathies. Thromb Res. 2014;133 Suppl 2:S70-S5.
- Levi M. Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol. 2009;22(1):129-36.
- Feinstein DI. Disseminated intravascular coagulation in patients with solid tumors. Oncology (Williston Park, NY). 2015;29(2):96-102.
- Kwaan HC, Gordon LI. Thrombotic microangiopathy in the cancer patient. Acta Haematol. 2001;106(1-2):52-6.
- Sack GH, Jr., Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Levine MN. Prevention of thrombotic disorders in cancer patients undergoing chemotherapy. Thromb Haemost. 1997;78(1):133-6.
- Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist. 2003;8(4):381-8.
- Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore). 1999;78(5):285-91.
- Zangari M, Anaissie E, Barlogie B, Badros A, Desikan R, Gopal AV, et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood. 2001;98(5):1614-5.
- Desai AA, Vogelzang NJ, Rini BI, Ansari R, Krauss S, Stadler WM. A high rate of venous thromboembolism in a multi-institutional phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil and daily thalidomide in patients with metastatic renal cell carcinoma. Cancer. 2002;95(8):1629-36.
- Kuenen BC, Levi M, Meijers JC, Kakkar AK, van H, V, Kostense PJ, et al. Analysis of coagulation cascade and endothelial cell activation during inhibition of vascular endothelial growth factor/vascular endothelial growth factor receptor pathway in cancer patients. Arterioscler Thromb Vasc Biol. 2002;22(9):1500-5.
- Kuenen BC, Rosen L, Smit EF, Parson MR, Levi M, Ruijter R, et al. Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol. 2002;20(6):1657-67.
- Straub PW. Diffuse intravascular coagulation in liver disease?. [Review] [65 refs]. Seminars in Thrombosis & Hemostasis. 1977;4(1):29-39.
- Levi M. Disseminated intravascular coagulation: a disease-specific approach. Semin Thromb Hemost. 2010;36(4):363-5.
- Seligsohn U. Disseminated Intravascular Coagulation. In: Handin RI, Lux SE, Stossel TP, editors. Blood: Pinciples and Practice of Hematology. Philadelphia: J.B.Lippingcott; 2000.
- Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: a review. Am Heart J. 1987;113(3):773-84.
- Donati MB. Cancer and thrombosis: from Phlegmasia alba dolens to transgenic mice. [Review] [32 refs]. Thrombosis & Haemostasis. 1995;74(1):278-81.
- Hyman DM, Soff GA, Kampel LJ. Disseminated intravascular coagulation with excessive fibrinolysis in prostate cancer: a case series and review of the literature. Oncology. 2011;81(2):119-25.
- Levi M, Scully M, Singer M. The role of ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost. 2018.
- Tallman MS. The thrombophilic state in acute promyelocytic leukemia. [Review] [72 refs]. Seminars in Thrombosis & Hemostasis. 1999;25(2):209-15.
- Rodeghiero F, Castaman G. The pathophysiology and treatment of hemorrhagic syndrome of acute promyelocytic leukemia. Leukemia. 1994;8 Suppl 2:S20-S6.
- Stein E, McMahon B, Kwaan H, Altman JK, Frankfurt O, Tallman MS. The coagulopathy of acute promyelocytic leukaemia revisited. Best practice & research Clinical haematology. 2009;22(1):153-63.
- Mitrovic M, Suvajdzic N, Elezovic I, Bogdanovic A, Djordjevic V, Miljic P, et al. Thrombotic events in acute promyelocytic leukemia. Thromb Res. 2015;135(4):588-93.
- Levi M, Scully M. How I treat disseminated intravascular coagulation. Blood. 2017.
- Thachil J, Falanga A, Levi M, Liebman H, Di Nisio M. Management of cancer-associated disseminated intravascular coagulation: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13(4):671-5.
- Levi M. Management of cancer-associated disseminated intravascular coagulation. Thromb Res. 2016;140 Suppl 1:S66-70.
- Marti-Carvajal AJ, Anand V, Sola I. Treatment for disseminated intravascular coagulation in patients with acute and chronic leukemia. The Cochrane database of systematic reviews. 2015(6):Cd008562.
- Thachil J, Falanga A, Levi M, Liebman H, Di Nisio M. Management of cancer-associated disseminated intravascular coagulation: guidance from the SSC of the ISTH: reply. J Thromb Haemost. 2015.
- Kakkar AK, Kadziola Z, Williamson RC. Low molecular weight heparin therapy and survival in advanced cancer. Blood. 2002;100:148a (abstract).
- Schunemann HJ, Ventresca M, Crowther M, Briel M, Zhou Q, Garcia D, et al. Use of heparins in patients with cancer: individual participant data meta-analysis of randomised trials study protocol. BMJ open. 2016;6(4):e010569.
- Di Minno MND, Ageno W, Lupoli R, Conte G, van Es N, Buller HR, et al. Direct oral anticoagulants for the treatment of acute venous thromboembolism in patients with cancer: a meta-analysis of randomised controlled trials. Eur Respir J. 2017;50(3).
- Niers TM, Klerk CP, DiNisio M, Van Noorden CJ, Buller HR, Reitsma PH, et al. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Critical reviews in oncology/hematology. 2007;61(3):195-207.
- Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10(4):222.
- Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356(22):2301-11.
- Avvisati G, ten Cate JW, Buller HR, Mandelli F. Tranexamic acid for control of haemorrhage in acute promyelocytic leukaemia. Lancet. 1989;2(8655):122-4.
