As the clinical evidence keeps on accumulating, we can clearly state that coronavirus disease 2019 (COVID-19) very often shows features of a coagulation disease/disorder.1 This association poses important consequences in the management of both patients suffering from COVID-19, and of patients who were affected by thrombotic and thromboembolic disease before the onset of the pandemic.
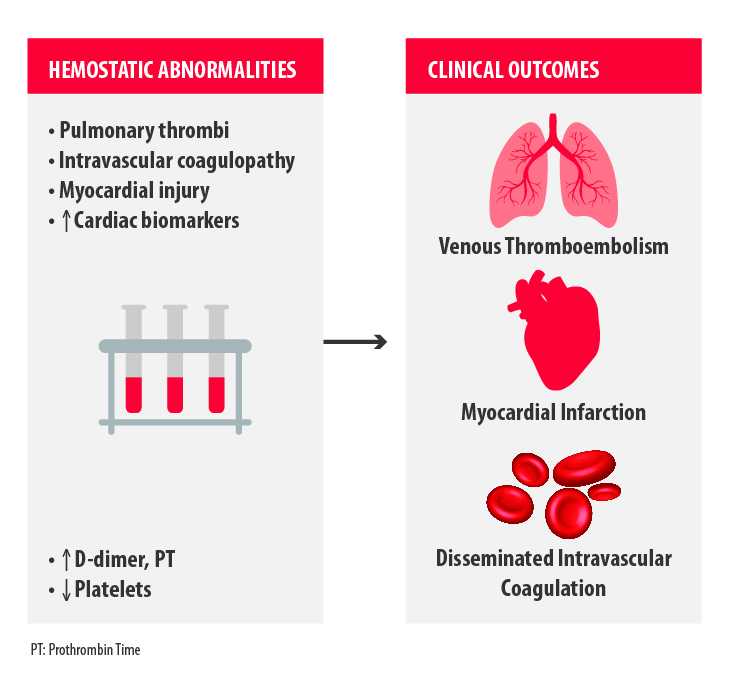
The major clinical outcomes observed in severely ill COVID-19 patients related to hemostasis disorders are venous thromboembolism, myocardial infarction and disseminated intravascular coagulation (Figure 1)2.
How does COVID-19 affect the onset of thrombotic/thromboembolic disease? The exact mechanism by which the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus induces thrombotic events is yet to be fully understood and the concomitant intervention of indirect factors may play a role.
Some of the consequences of the SARS-CoV-2 infection that predispose to thrombotic complications are:
- Severe illness and hypoxia
- Severe inflammatory response
- Experimental therapies that may cause adverse drug–drug interactions with antiplatelet agents and anticoagulants
- Unbalanced allocation of resources, which may result in neglecting the care of patients without COVID-19 but with thrombotic events
In particular, the dysregulated inflammatory response, which has been described in COVID-19 acute cases (often referred as “cytokine storm”), could activate coagulation pathways, and this results in a double effect3:
- Inducing thrombi formation
- Amplifying the inflammatory signals, in a self-generating loop, which will keep fueling adverse events
How can a high-risk thrombotic situation be promptly identified? Which are the most common parameters that must be assessed with priority?
Even if it is not clear whether these changes in marker levels are a specific effect of SARS-CoV-2 or just a consequence of the cytokine storm, healthcare professionals need some specific points to focus on to guide their intervention.
Despite the general worldwide decrease in the hospitalization burden, the need of an unequivocal way to assess patients at risk is still urgent as the pandemic is far to be a mere memory.
The thrombosis and hemostasis parameters identified to be associated with poor COVID-19 outcome that should be checked at hospital admission are:
- D-dimer levels, that are often increased4
- Thrombocytopenia5
- Lymphopenia6
- Prolongation of the prothrombin time7
- Thrombin time8
- Activated partial thromboplastin time9
- Troponin levels as a marker for myocardial injury10
A guidance on recognition and management of coagulopathy in COVID-19 was also proposed earlier in the pandemic by the International Society of Thrombosis and Haemostasis (ISTH).11
In addition to markers for coagulation disorders, common laboratory abnormalities found in patients with COVID-19 that would need special attention (also in relation to the tight relation between coagulation and inflammation) are:
- Lactate dehydrogenase7
- Inflammatory markers (C reactive protein, ferritin and interleukin-6)7
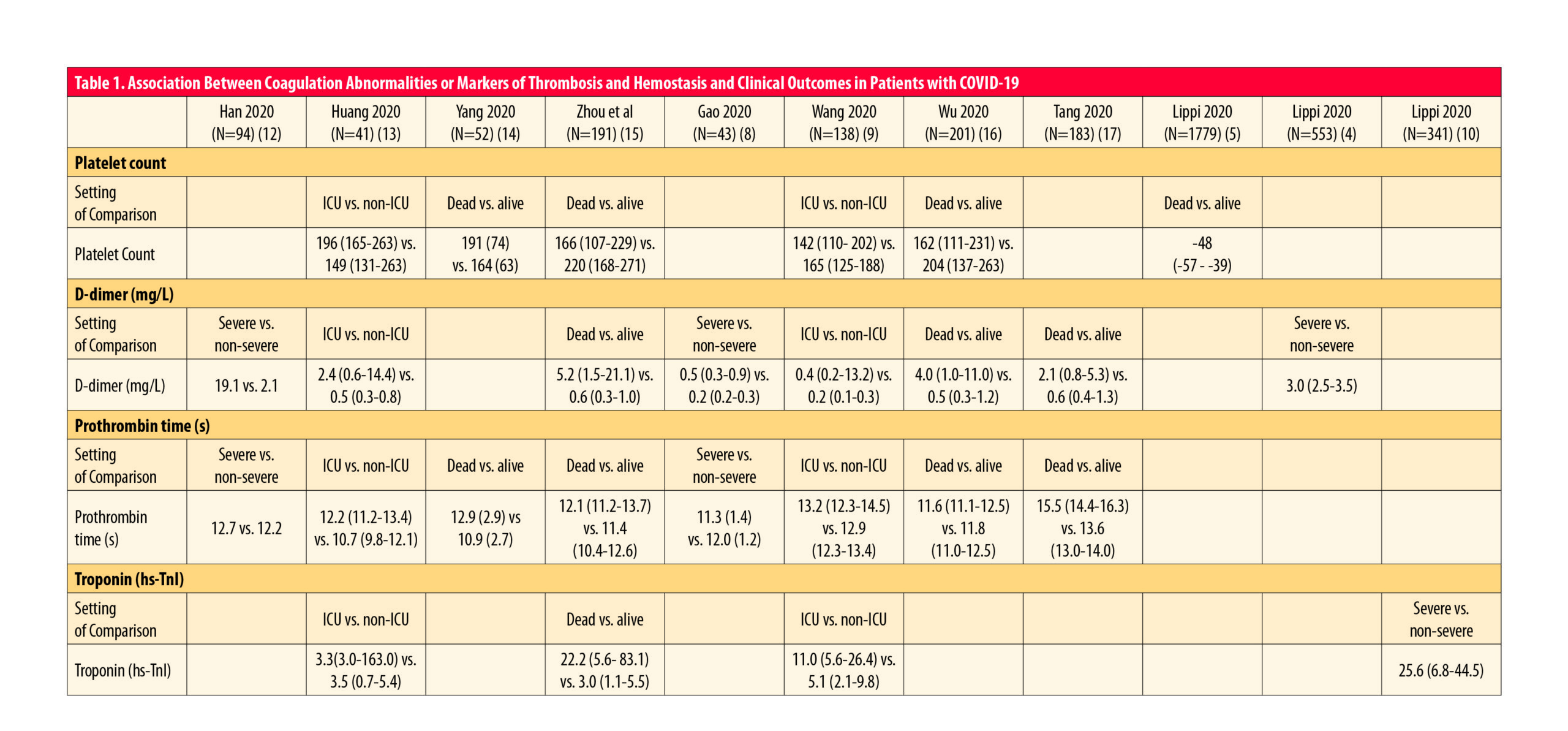
To summarize the association between markers of thrombosis and hemostasis and clinical outcomes in patients with COVID-19, see Table 1 (adapted from2).
While there is a general agreement for some of the parameters, for others there are still some contradictory issues (such as for activated partial thromboplastin time). Nevertheless, it is important not to ignore any marker a priori, and to see the complete picture of the situation.
Data are rapidly accumulating from clinical evidence and combined with the analysis of the follow-up time, it will be feasible to soon adjust these general indications.
References
- Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;S2352-3026(20)30145-9. doi:10.1016/S2352-3026(20)30145-9
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;S0735-1097(20)35008-7. doi:10.1016/j.jacc.2020.04.031
- Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;S2213-2600(20)30216-2. doi:10.1016/S2213-2600(20)30216-2
- Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(5):876‐878. doi:10.1055/s-0040-17096509
- Lippi G, Plebani M, Michael Henry B. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta 2020. doi:10.1016/j.cca.2020.03.022
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. doi:10.1038/s41586-020-2012-7
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. doi:10.1016/S0140-6736(20)30566-3
- Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;10.1002/jmv.25770. doi:10.1002/jmv.25770
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. doi:10.1001/jama.2020.1585
- Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020. doi:10.1016/j.pcad.2020.03.001
- Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023‐1026. doi:10.1111/jth.14810
- Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection [published online ahead of print, 2020 Mar 16]. Clin Chem Lab Med. 2020;/j/cclm.ahead-of-print/cclm-2020-0188/cclm-2020-0188.xml. doi:10.1515/cclm-2020-0188
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:]. Lancet. 2020;395(10223):497‐506. doi:10.1016/S0140-6736(20)30183-5
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26]. Lancet Respir Med. 2020;8(5):475‐481. doi:10.1016/S2213-2600(20)30079-5
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet. 2020;395(10229):1054‐1062. doi:10.1016/S0140-6736(20)30566-3
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China [published online ahead of print, 2020 Mar 13]. JAMA Intern Med. 2020;e200994. doi:10.1001/jamainternmed.2020.0994
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. doi:10.1111/jth.14768