In 1865, Armand Trousseau famously described unexplained thrombotic events as a first manifestation of occult malignancy[1]. His documented cases mainly concerned venous thromboembolic events such as migratory thrombophlebitis[2]. Accordingly, the medical community has traditionally focused on the association between venous thromboembolism (VTE) and cancer[3]. This association has been well established, with studies demonstrating that about one in 20 patients will be diagnosed with a malignancy within a year after VTE[4]. Meanwhile, the association with arterial thromboembolism (ATE) has been largely overlooked. In fact, it was only in the last decade that large-scale, population-based studies established the increased risk of ATE after cancer diagnosis[5–7]. In these studies, the highest risk was observed early after cancer diagnosis. If cancer directly induces thrombosis, it is reasonable to expect that the risk of ATE is also increased prior to diagnosis. Alternatively, there might be external factors that explain the increased risk, including treatment (e.g. chemotherapy) and even mental stress.
A recently published American study has shed light upon the question whether arterial thromboembolism can be a first manifestation of cancer[8]. In the study, the incidence of first myocardial infarction or ischemic stroke within the year preceding cancer diagnosis was assessed retrospectively. Oncological patients were matched with cancer-free controls for demographics and comorbidities, amounting to a population of more than 700,000 participants. Usage of the Surveillance Epidemiology and End Results (SEER) database restricted the study to an elderly population on Medicare. The researchers included nine types of cancer (breast, lung, prostate, colorectal, bladder, non-Hodgkin lymphoma, uterine, pancreatic and gastric), covering about two-thirds of all cancer incidence. Their results demonstrate that risk of ATE first increased 5 months before cancer diagnosis and progressively increased towards the date of diagnosis, culminating in a fivefold increased risk during the last month.
A major limitation of this study is the lack of information on shared risk factors between ATE and cancer, not least the omission of smoking prevalence. However, these factors cannot explain the crescendo phenomenon observed prior to cancer diagnosis. The risk was also increased among all studied cancer types, some of which are not associated with smoking. Further refuting the importance of shared risk factors are the results of a prospective study on the association between myocardial infarction and subsequent cancer. The study reported that adjustment for several risk factors had no influence on the risk[9].
Another drawback of the SEER study design that the researchers point out, is that it cannot determine to what extent increased medical surveillance after arterial thromboembolism contributed to a timely cancer diagnosis. Moreover, arterial thromboembolism might also have occurred as a result of interruption of antithrombotic therapy so that a biopsy of a newly discovered mass could be performed. Nonetheless, it is plausible that the malignancy itself has a leading role in the development of ATE. After all, as the researchers underscored, the clear correlation with cancer stage suggests a biological gradient between cancer activity and ATE risk.
Cancer is understood to induce a prothrombotic phenotype through various pathogenic mechanisms of which individual contributions are uncertain. These include the expression of procoagulants (e.g. tissue factor), anti-fibrinolytics (e.g. PAI-1), cell adhesion molecules (e.g. P-selectin), and the release of microvesicles[10]. Some adenocarcinomas carry an exceptionally high risk of VTE, which is attributed to the secretion of aberrantly glycosylated mucins that indirectly activate platelets[10]. Cancer may also stimulate the formation of neutrophil extracellular traps (NETs). This effect might prove particularly interesting since circumstantial evidence has linked NETs to myocardial infarction and ischemic stroke in occult cancer[11].
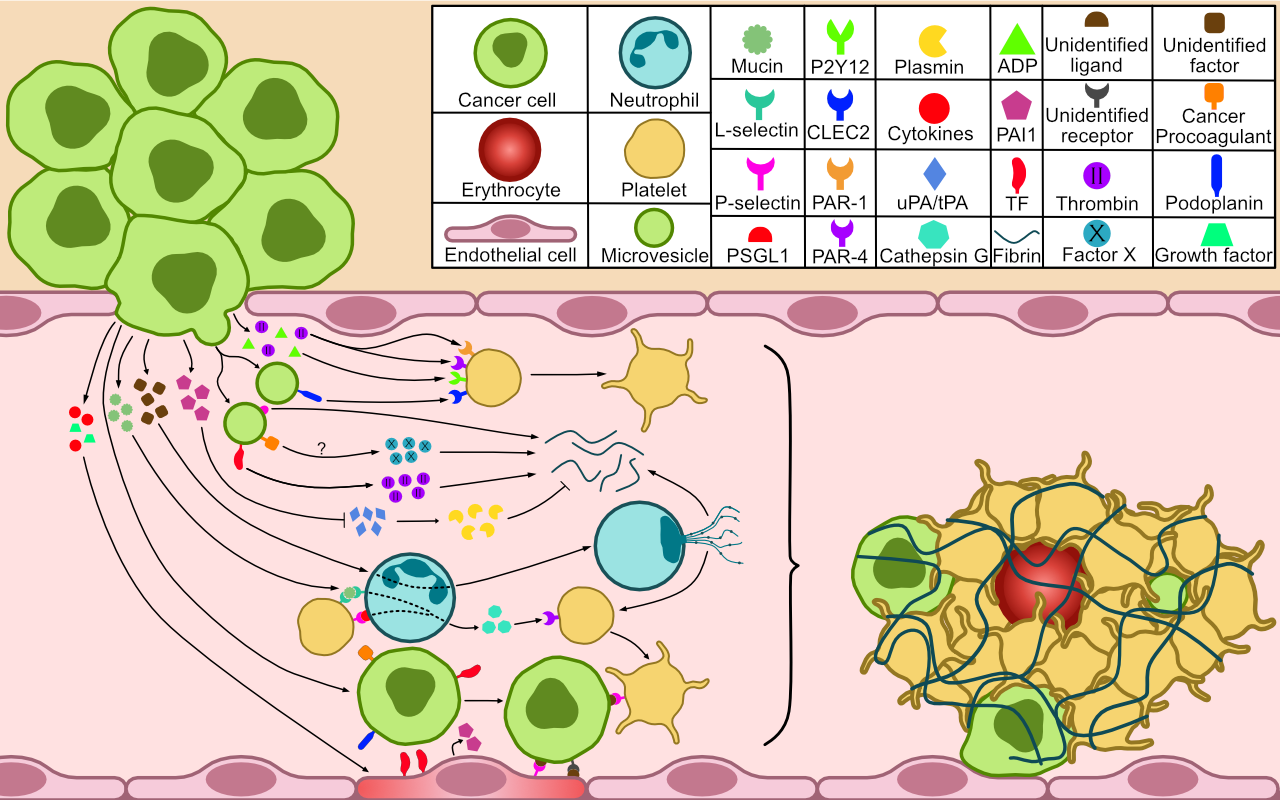
It is remarkable that the subset of malignancies that are most strongly associated with ATE corresponds to that of VTE[12]. Although this suggests a significant pathogenetic link, the differences between thrombus formation in the arterial and venous systems are substantial. In the SEER study, the relative increases in myocardial infarction and ischemic stroke were similar[8]. The increased risk of occult cancer in lower limb arterial thrombosis has also been established[13]. However, these studies do not report on the underlying etiology of these events. These may range from local thrombosis due to tumor invasion or compression to systemic embolism. It cannot simply be assumed that the etiology is similar to that described in ATE after cancer diagnosis, considering that those patients have been exposed to cancer therapy and other external factors[14]. A retrospective study on the etiology of ischemic stroke as the first manifestation of occult cancer found a strikingly high frequency of non-bacterial thrombotic endocarditis[15]. These valvular vegetations are a source of systemic emboli and are thought to result from a hypercoagulable state, most commonly associated with advanced and mucin-producing tumors[16]. This underlines the necessity to evaluate ATE etiology in occult cancer.
Nonetheless, it can reasonably be assumed that atherosclerosis is a major cause of ATE in cancer[12]. Atherosclerosis results in clinical events through plaque rupture or endothelial erosion. The chronic inflammatory response in cancer might trigger plaque disruption[17,18]. At the moment of plaque disruption, the aforementioned disturbances of thrombogenic and fibrinolytic potential might further predispose to the development of clinically relevant atherothrombosis[19]. Besides, it has been established that carcinogenesis and atherosclerosis share pathogenic pathways and are both stimulated by chronic inflammation[20,21]. Thus, cancer and atherosclerosis might develop simultaneously based on common dysregulations. However, considering the observed close temporal association between ATE and subsequent cancer diagnosis, it is unlikely to explain the presence of concurrent occult cancer. Even so, it could be an important factor in explaining the long-term increased risk of ATE after cancer[22].
If ATE is a possible first manifestation of cancer, then a discussion on cancer screening needs to be held. This discussion can be expected to unfold in a similar manner to that of VTE. Studies on extensive screening in VTE failed to show an increase in occult cancer detection or improvement in patient outcomes[23,24]. The same, negative, results of unselected screening in ATE might be expected, with a study reporting that a major percentage of cancers was detected without screening after myocardial infarction[9]. Studies also show a poor outcome for cancer detected after ATE and in the SEER study almost half of cancers were of advanced stage[8,15]. Current consensus states that only limited screening should be performed after unprovoked VTE. Similarly, cancer screening might currently only be warranted after ATE in patients with low cardiovascular risk. However, there are likely subgroups of patients with a higher risk of occult cancer detection in which extensive screening is warranted. Verified risk models and biomarkers are needed to identify this subgroup[25].
References
- Trousseau A. Phlegmasia alba dolens. Lectures on clinical medicine, delivered at the Hotel-Dieu, Paris, 3rd ed. (JR Cormack, trans). London: The New Sydenham Society, 1872, p. 281-332.
- Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007; 110(6): 1723-9.
- De Stefano V. Arterial thrombosis and cancer: the neglected side of the coin of Trousseau syndrome. Haematologica. 2018; 103(9): 1419-21.
- van Es N, Le Gal G, Otten HM, Robin P, Piccioli A, Lecumberri R, et al. Screening for occult cancer in patients with unprovoked venous thromboembolism: a systematic review and meta-analysis of individual patient data. Ann Intern Med. 2017; 167(6): 410-7.
- Zoller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012; 48(12): 1875-83.
- Zoller B, Ji J, Sundquist J, Sundquist K. Risk of coronary heart disease in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012; 48(1): 121-8.
- Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017; 70(8): 926-38.
- Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Tagawa ST, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. 2019; 133(8): 781-9.
- Rinde LB, Smabrekke B, Hald EM, Brodin EE, Njolstad I, Mathiesen EB, et al. Myocardial infarction and future risk of cancer in the general population-the Tromso Study. Eur J Epidemiol. 2017; 32(3): 193-201.
- Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers. 2018; 10(10): pii: E380.
- Thalin C, Demers M, Blomgren B, Wong SL, von Arbin M, von Heijne A, et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res. 2016; 139: 56-64.
- Oren O, Herrmann J. Arterial events in cancer patients-the case of acute coronary thrombosis. J Thorac Dis. 2018; 10(Suppl 35): S4367-S85.
- Sundboll J, Veres K, Horvath-Puho E, Adelborg K, Sorensen HT. Risk and prognosis of cancer after lower limb arterial thrombosis. Circulation. 2018; 138(7): 669-77.
- Aronson D, Brenner B. Arterial thrombosis and cancer. Thromb Res. 2018; 164(Suppl 1): S23-S28.
- Taccone FS, Jeangette SM, Blecic SA. First-ever stroke as initial presentation of systemic cancer. J Stroke Cerebrovasc Dis. 2008; 17(4): 169-74.
- el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007; 12(5): 518-23.
- Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011; 144(5): 646-74.
- Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015; 278(5): 483-93.
- Newby DE. Triggering of acute myocardial infarction: beyond the vulnerable plaque. Heart. 2010; 96(15): 1247.
- Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer. Ann NY Acad Sci. 2001; 947(1): 271-93.
- Morganti M, Carpi A, Nicolini A, Gorini I, Glaviano B, Fini M, et al. Atherosclerosis and cancer: common pathways on the vascular endothelium. Biomed Pharmacother. 2002; 56(7): 317-24.
- Jang H-S, Choi J, Shin J, Chung J-W, Bang OY, Kim G-M, et al. The long-term effect of cancer on incident stroke: a nationwide population-based cohort study in Korea. Front Neurol. 2019; 10: 52.
- Carrier M, Lazo-Langner A, Shivakumar S, Tagalakis V, Zarychanski R, Solymoss S, et al. Screening for occult cancer in unprovoked venous thromboembolism. New Engl J Med. 2015; 373(8): 697-704.
- Robin P, Le Roux P-Y, Planquette B, Accassat S, Roy P-M, Couturaud F, et al. Limited screening with versus without 18F-fluorodeoxyglucose PET/CT for occult malignancy in unprovoked venous thromboembolism: an open-label randomised controlled trial. Lancet Oncol. 2016; 17(2): 193-9.
- Robin P, Carrier M. Revisiting occult cancer screening in patients with unprovoked venous thromboembolism. Thromb Res. 2018; 164: S7-S11.
