Cancer is the second most common cause of death in children in developed countries. Fortunately, survival rates have improved enormously over the last decades: the 5-year survival rates have increased from less than 40% in the 1970s to almost 80% in the last decade due to better diagnostic procedures, multimodal treatment strategies and improved supportive care.
However, the enlarged intensity of the primary treatment results in more frequent severe side effects, including myelosuppression, infection, and venous thromboembolism (VTE).
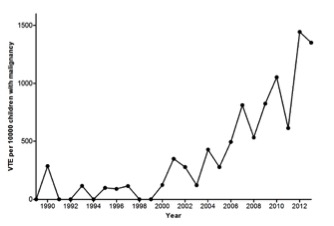
VTE has become increasingly diagnosed in children in general and the incidence of VTE appeared to have augmented over time in children with cancer, as well. Recently, one single center showed a 13-fold increase in incidence of VTE in pediatric oncology patients in a 25 years period[1].
Overall, the incidence of VTE in children with cancer varies between 2.1 and 50%, depending on the type of malignancy, the study design and inclusion of asymptomatic thrombi[2]. Most thrombi occur in children with hematological malignancies, in particular acute lymphoblastic leukemia (ALL) and lymphoma, and in children with sarcomas. The incidence of symptomatic VTE in ALL patients is about 7%. VTE may lead to considerable mortality and morbidity.
In ALL patients with sinovenous thrombosis, the mortality rate is reported to be between 0 and 28%. Morbidity may include recurrent thrombosis, neurologic changes after sinovenous thrombosis, catheter removal, bleeding due to antithrombotic agents, and the development of the post thrombotic syndrome. Furthermore, in ALL patients, VTE may lead to suboptimal therapy, due to the necessity to interrupt, delay, or even discontinue chemotherapy (i.e. asparaginase), and thereby to inferior disease outcomes.
Prevention of VTE will decrease mortality and morbidity and further improve cancer survival rates. Thromboprophylaxis may be warranted in children with cancer and the highest risk for VTE. Therefore, it is important to identify the most significant risk factors for VTE in children with cancer.
As the majority of VTE occur in ALL patients, most studies about risk factors have been performed in children with ALL. The pathogenesis of VTE is not entirely clear in these patients. Typically, several factors contribute to the development of thrombosis[3,4]. Almost all thrombi occur during administration of asparaginase, usually in combination with corticosteroids. Asparaginase depletes the asparagine pool by catalyzing the hydrolysis of asparagine to aspartic acid and ammonia. Asparagine deficiency causes reduction of protein synthesis and cell proliferation resulting in cytotoxicity of the lymphoblasts. One of the side effect includes diminished levels of both coagulation factors and anticoagulant proteins, especially antithrombin, and upregulated levels of tissue factor causing thrombin initiation. Steroids enhance the hypercoagulable state by elevation of factor VIII/von Willebrand factor complex and by inducing a hypofibrinolytic state due to increased plasminogen activator inhibitor 1.
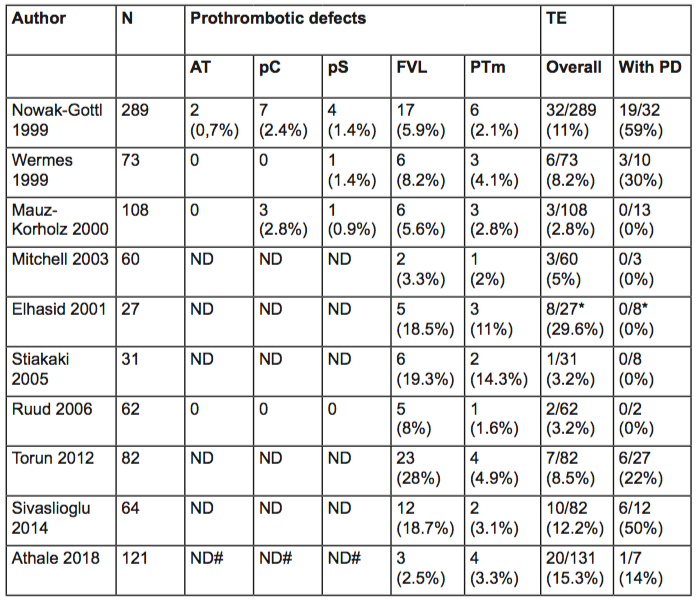
In addition to chemotherapy, therapy-related factors including central venous catheters and infection contribute to the increased risk of thrombosis in children with ALL. Furthermore, host-associated factors may influence the risk of VTE, such as age, non-O blood group and the presence of congenital thrombophilia. Large meta-analyses have shown the contribution of congenital thrombophilia, including deficiencies of antithrombin, protein C and S, and the presence of factor V Leiden and factor II mutation to the development of VTE in otherwise healthy population[5]. Literature about the extent of this contribution to the development of VTE in children with ALL is scarce and show conflicting results (Table 1).
Reports show wide variability in both the reported prevalence of thrombophilia and the frequency of ALL-associated VTE in children with thrombophilia, reflecting the different ethnicity of the population studied, the variability in the extent of thrombophilia tested, the different treatment protocols used, and the small sample sizes.
In summary, most studies do not support an association between congenital thrombophilia with increased risk of symptomatic VTE. It might be possible that the prothrombotic effects of the disease itself in combination with specific chemotherapy and central venous catheters prevail over the effect of the inherited prothrombotic risk factors.
However, prospective studies with a larger sample size are needed to confirm this hypothesis. Until then, routine screening of congenital prothrombotic defects in pediatric cancer patients should not be performed.
References
- Klaassen ILM, van Els AL, van de Wetering MD, van Ommen CH. Increasing Incidence and Recurrence Rate of Venous Thromboembolism in Paediatric Oncology Patients in One Single Centre Over 25 Years. Thromb Haemost. 2017;117(11):2156-62.
- van Ommen CH, Chan AK. Supportive care in pediatric cancer: the road to prevention of thrombosis. Semin Thromb Hemost. 2014;40(3):371-81.
- Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia. Part II. Pathogenesis of thrombosis in children with acute lymphoblastic leukemia: effects of the disease and therapy. Thromb Res. 2003;111(4-5):199-212.
- Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia Part III. Pathogenesis of thrombosis in children with acute lymphoblastic leukemia: effects of host environment. Thromb Res. 2003;111(6):321-7.
- Young G, Albisetti M, Bonduel M, Brandao L, Chan A, Friedrichs F, et al. Impact of Inherited Thrombophilia on Venous Thromboembolism in Children. A Systematic Review and Meta-Analysis of Observational Studies. Circulation. 2008;118(13):1373-82.
