Patients with cancer are at high risk of venous thromboembolism (VTE) recurrence and major bleeding (1). Most guidelines recommend a minimum of six months of anticoagulant treatment (2-4). Still, there is no consensus on the anticoagulant therapy of patients with cancer-associated thrombosis (CAT), especially after six months. Even though most guidelines recommend extending anticoagulant treatment in patients with CAT during the entire antineoplastic therapies, the decision is made on a case by case basis.
Some studies also suggest that anticoagulant therapy beyond six months may reduce the risk of VTE recurrence and bleeding. The administration of dalteparin therapy beyond six months was associated with a low bleeding rate (5). Treatment with tinzaparin beyond six months was safe and reduced the incidence of clinically relevant bleedings and recurrences (6). However, these studies do not provide definite indications for the management of CAT patients.
Two extensive studies –the PREDICARE and the aXa– describe CAT patients’ treatment with tinzaparin for six months. 432 patients with cancer and diagnosed acute VTE included in the PREDICARE and aXa studies were recruited to participate in the Usual Care of Cancer-Associated Thrombosis (USCAT) study, a retrospective non-interventional multicenter cohort study (7).
The USCAT study describes the anticoagulant treatment for managing CAT patients and their clinical outcomes up to 12 months after the index VTE. All the patients were previously treated for six months with tinzaparin. The majority of the CAT patients have been prescribed a further anticoagulant treatment for a median duration of four to five months.
The study population’s mean age was 66.5 ± 12.7 years, and 92% of patients had one cancer site, mostly a solid tumor. Breast, colorectal, and lung were the most represented cancers. The index VTE diagnosis was in 74% of cases a pulmonary embolism (either isolated or combined with thrombosis) and 26% of cases deep vein thrombosis.
The most used ongoing anticoagulant treatment was low-molecular-weight heparin, in 74% of patients, followed by vitamin K, direct oral anticoagulant, unfractionated heparin, and fondaparinux.
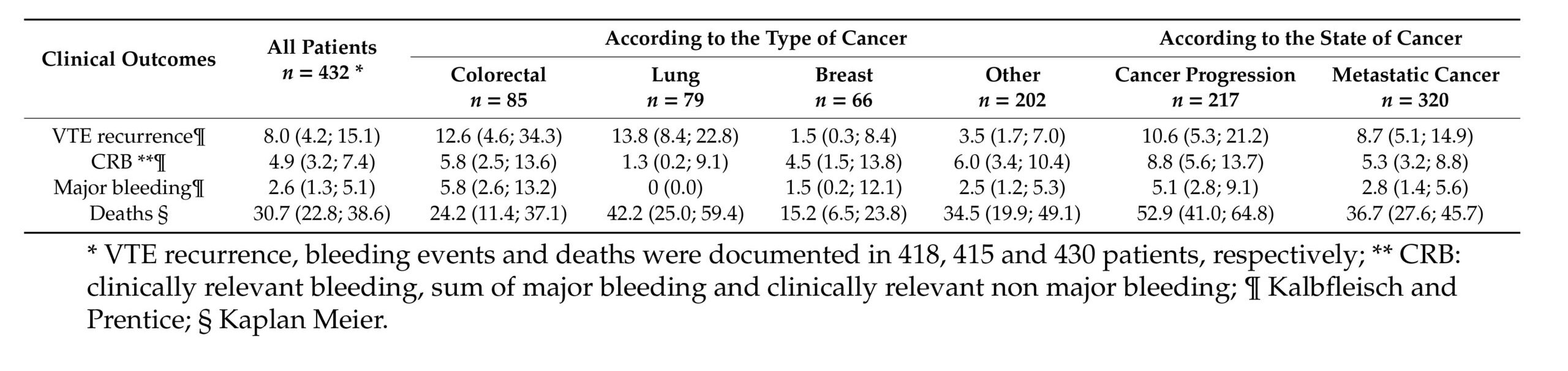
The study highlights how the cancer site influences the clinical profile of the VTE-related outcomes—patients with lung and colorectal cancer experience higher VTE recurrence, with the latter also experiencing major bleeding (Tab. 1). Previous studies already reported higher rates of VTE and major bleeding during anticoagulant therapy for patients with colorectal and lung cancer. Gastrointestinal cancer was correlated with an increased risk of major bleeding (8,9), while lung cancer with a higher rate of thromboembolic recurrences (10).
These results call for precautions when using an anticoagulated treatment in these patients and highlight the need to develop targeted therapy.
The USCAT study also suggests a close relationship between the stage and progression of cancer and the risk of VTE recurrence. These data suggest extending anticoagulant therapy for as long as the cancer is active, or the patient receives an antineoplastic treatment.
One limitation of the USCAT study is the lack of randomization and concurrent control. However, this study has the largest sample size of CAT patients enrolled after a six-month treatment with LMWH in an observational study. It also has a relatively large number of patients having completed the 12-month follow-up. This study’s strength is the use of a competitive risk method to estimate outcomes of cumulative incidences and no ambivalence clause.
In summary, the USCAT study identified cancer types associated with VTE recurrence or bleeding, which can provide a guideline for the management of anticoagulant treatment in patients with CAT. Special attention needs to be put on the type of cancer, its stage, and progression for the treatment of patients with CAT beyond six months.
This article has been sponsored by an unrestricted educational grant from LEO Pharma A/S.
References
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant Treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484-3488. doi:10.1182/blood-2002-01-0108
- Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566-e581. doi:10.1016/S1470-2045(19)30336-5
- Key NS, Khorana AA, Kuderer NM, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38(5):496-520. doi:10.1200/JCO.19.01461
- Sanchez O, Benhamou Y, Bertoletti L, et al. Recommandations de bonne pratique pour la prise en charge de la maladie veineuse thromboembolique chez l’adulte – Version longue [Recommendations for best practice in the management of venous thromboembolic disease in adults. Long version] [published online ahead of print, 2019 Jul 4]. Rev Mal Respir. 2019;S0761-8425(19)30210-4. doi:10.1016/j.rmr.2019.05.038
- Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost. 2015;13(6):1028-1035. doi:10.1111/jth.12923
- Jara-Palomares L, Solier-Lopez A, Elias-Hernandez T, et al. Tinzaparin in cancer associated thrombosis beyond 6months: TiCAT study. Thromb Res. 2017;157:90-96. doi:10.1016/j.thromres.2017.07.004
- Mahé I, Plaisance L, Chapelle C, et al. Long-Term Treatment of Cancer-Associated Thrombosis (CAT) Beyond 6 Months in the Medical Practice: USCAT, a 432-Patient Retrospective Non-Interventional Study. Cancers (Basel). 2020;12(8):2256. Published 2020 Aug 12. doi:10.3390/cancers12082256
- Kraaijpoel N, Di Nisio M, Mulder FI, et al. Clinical Impact of Bleeding in Cancer-Associated Venous Thromboembolism: Results from the Hokusai VTE Cancer Study. Thromb Haemost. 2018;118(8):1439-1449. doi:10.1055/s-0038-1667001
- Mahé I, Elalamy I, Gerotziafas GT, Girard P. Treatment of Cancer-Associated Thrombosis: Beyond HOKUSAI [published correction appears in TH Open. 2019 Nov 05;3(4):e348-e349]. TH Open. 2019;3(3):e309-e315. Published 2019 Sep 16. doi:10.1055/s-0039-1696659
- Mahé I, Chidiac J, Bertoletti L, et al. The Clinical Course of Venous Thromboembolism May Differ According to Cancer Site. Am J Med. 2017;130(3):337-347. doi:10.1016/j.amjmed.2016.10.017
