Patients with primary and metastatic brain tumors have a high risk of venous thromboembolism (VTE), occurring in 20-30% depending on primary tumor type [1-2]. Anticoagulant therapy for patients with brain tumors is challenging given concerns regarding risk of clinically significant intracranial hemorrhage (ICH), which occurs at a rate of between 2-20% in patients not receiving anticoagulation[3-4].
In order to evaluate the safety of anticoagulation with low molecular weight heparin (LMWH) in patients with brain tumors, we previously performed a matched cohort study to assess the incidence of ICH in patients with brain metastases and VTE treated with LMWH compared with similar patients not receiving therapeutic anticoagulation. We found no statistically significant difference between the LMWH and control groups in terms of 12-month cumulative incidence of major ICH (defined as volume greater than 10 mL, causing clinical symptoms, or requiring surgical intervention)[3].
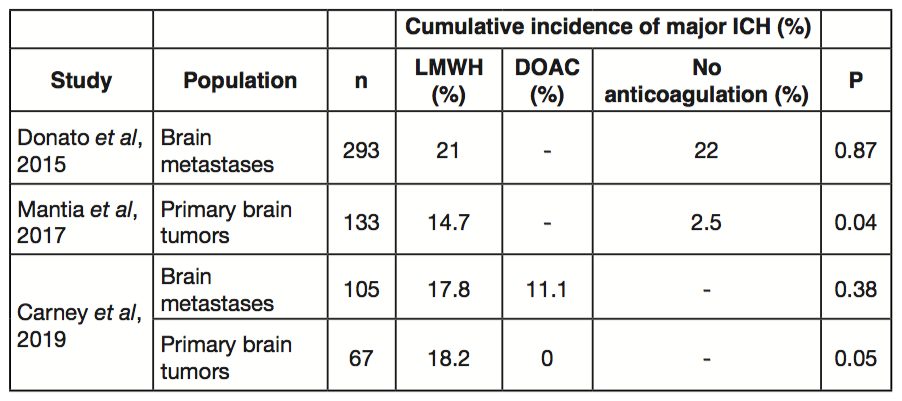
In a similarly designed study only evaluating glioma cohorts, we observed that LMWH did not increase the 12-month cumulative incidence of all ICH compared to no anticoagulation (Table 1). However, LMWH was associated with a greater than three-fold increased risk of major ICH (14.7% vs. 2.5%, P=0.036)[4]. These data suggest that anticoagulation with LMWH does not increase the risk of major hemorrhage in the setting of brain metastases but should be approached with caution in patients with primary brain tumors such as glioblastoma multiforme.
There is a growing body of data supporting the efficacy of direct oral anticoagulants (DOACs) for cancer-associated thrombosis[5-6]. DOACs have been associated with increased risk of bleeding in certain populations, most notably patients with upper gastrointestinal and possibly genitourinary primary tumors. In order to specifically focus on the incidence of ICH with DOAC in cancer patients with brain tumors, we compared safety outcomes between cohorts who received therapeutic LMWH versus a DOAC[7]. In this retrospective cohort study of 105 patients with brain metastases, we found no statistically significant difference in the 12-month cumulative incidence of ICH between DOACs and LMWH. Notably, among the 20 patients with primary brain tumors treated with a DOAC, we did not observe a single major ICH. In both the brain metastases and primary brain tumor cohorts, patients receiving DOACs were more likely to have additional risk factors for hemorrhage (e.g. hypertension, chronic kidney disease, and concomitant aspirin use) suggesting that the safety of DOACs was not due to confounding.
In light of increasing use of DOAC for the treatment of cancer-associated thrombosis, it is reassuring that emerging data with DOAC do not identify an increased risk of ICH among patients with primary and secondary brain tumors.
References
- Weinstock MJ, Uhlmann EJ, Zwicker JI. Intracranial hemorrhage in cancer patients treated with anticoagulation. Thromb Res. 2016; 140 Suppl 1: S60-5.
- Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol. 2006; 24(8): 1310-8.
- Donato J, Campigotto F, Uhlmann EJ, et al. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood. 2015; 126(4): 494-9.
- Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017; 129(25): 3379-3385.
- Raskob GE, Van es N, Verhamme P, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2017; 378(7): 615-624.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an Oral Factor Xa Inhibitor with Low Molecular Weight Heparin in Patients with Cancer with Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol. 2018; 36(20): 2017-23.
- Carney BJ, Uhlmann EJ, Puligandla M, et al. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J Thromb Haemost. 2019; 17(1): 72-76.
