The normal experience of cancer patients who receive an implantable venous access device (Port-a-Cath) for chemotherapy infusion [Figure 1] is not well known, nor have the risk factors associated with thromboembolism been appropriately investigated. The general perception is that improvements in technology and methods of implantation of venous access catheters has reduced the frequency of thromboembolic events seen in past studies to the point where it is uncertain whether pharmacological protective measures are of any benefit, given that they have been shown to be generally ineffective for the prevention of upper-limb DVT which is directly attributable to the catheter. This view is shared by the authors of the main guidelines who in reality do not give a consistent recommendation of prophylaxis. But is this how things really are? No.
Figure 1. Port-a-Cath
Patients with an evolving cancer are at risk of major thromboembolism, which is not restricted to the risk of DVT induced directly by the catheter. Other risk factors are: systemic thromboembolism (both venous and arterial) which is sustained by hypercoagulability that most cancers (especially in the metastatic phase) induce; venous stasis accrued during periods of limited movement, such as during hospitalisation; advanced age; the presence of comorbidities; the result of surgical interventions or other aggressive manoeuvres which are indispensable for diagnostic or therapeutic purposes; personal or family predisposition which may be present and the infusion of chemotherapeutic agents associated per se – to a varying degree and impact – with an increase in thromboembolic risk.
So much so that in recent years scores have been identified and validated, including the Khorana risk score*[2,3] and a score using the Vienna prediction model[4], which help to predict which individuals have a higher thromboembolic risk. It is a subject which I dealt with at length in a recent article about the description of a simple algorithm proposed by the Austrian colleagues. The subject is therefore somewhat complex: it does not end with catheter-related risks and needs to be managed with a broad view and in consideration of the long-term prospects.
Let’s take a step back and consider the main question: what are the risks of a thromboembolic occurrence in patients today who require a Port-a-Cath for chemotherapy infusion?
This is the question that has been answered by the authors of an excellent prospective cohort study, ONCOCIP, which was conducted in France and limited to patients with solid neoplasms[1]. Decousus and colleagues investigated the follow-up of 3032 patients (median age 63 and 58% women) all of whom had a venous access implant and in over 40% of cases a solid, metastatic cancer. The investigation covered follow-up for up to 1 year from when the catheter was implanted.
For those patients (about a quarter) who died before the completion of the follow-up year, the observation was continued until death. The most frequent cancer locations were the breast (34%), the lung (18%) and colorectal (16%). At the time of being included in study, only 8% of patients were expected to have prophylactic doses of low molecular weight heparin and 15% antiplatelet therapy. It is therefore, a normal experience that the influence of antithrombotic drugs is minimal. Patients were examined each month for the first six months, and thereafter every three months until the completion of the one year of follow-up.
Comprehensive thromboembolic complications were documented in 397 patients (13.8%; 95%; CI, 12.6 -15.0) with steady growth over the entire follow-up period [Figure 2]. Symptomatic upper-limb catheter-related DVT developed in 111 patients (3.8%; 95%; CI, 3.2 – 4.5), of whom 5 had complications caused by pulmonary embolism. 25 patients (0.9%) developed arterial thromboembolic complications (ischemic stroke in 18, myocardial infarction in 5 and acute peripheral ischemia in 2), isolated or in association (in 6 cases) with VTE.
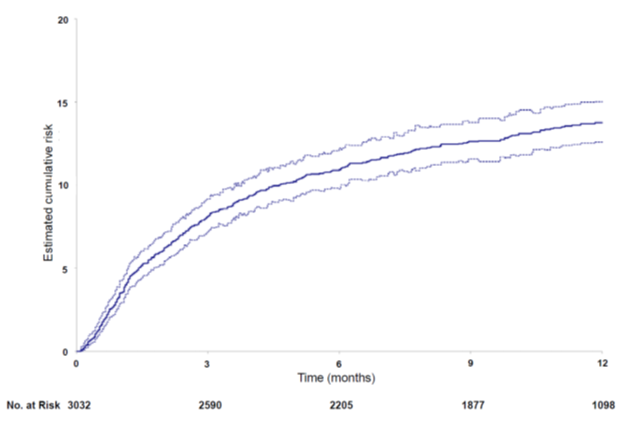
Figure 2. Cumulative incidence of thromboembolic complications during the 12-month follow-up
The only factor that was independently associated with the development of upper-limb catheter-related DVT was the use of the cephalic vein for catheter insertion (RR = 2.51; 95% CI: 1.68 – 3.75). This risk, on the other hand, was reduced by concomitant antiplatelet therapy (RR = 0.44; 95% CI: 0.21 – 0.90). Risk factors independently associated with the development of VTE in locations correlated with venous access catheters were > 60 years old, previous VTE, specific cancer location, anaemia (Hb <10 g / dL) and leukocytosis (leukocyte count > 11 cells / L) before the start of chemotherapy [Figure 3]. All parameters generally included in the Khorana score.*
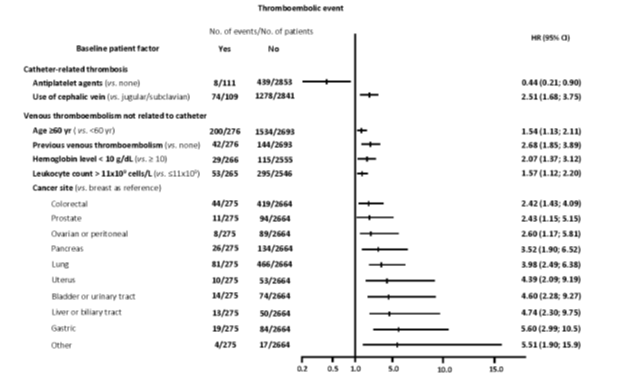
Figure 3. Predictive risk factors for the development of venous thromboembolic complications (multivariate analysis)
Comment
In the absence of adequate pharmacological protection, patients with solid cancers are exposed to a risk of systemic venous thromboembolic events and, to a lesser extent, catheter-related upper-limb DVT catheter as well as arterial thromboembolic complications. This risk starts from the time of the Port-a-Cath being implanted and lasts for at least one year (the period investigated by the French study). Consistent with the observations of the last five years, the risk of upper limb DVT is significantly increased by the use of the cephalic vein, which should therefore be avoided in cancer patients especially if there is a continuing lack of adequate preventative measures. The risk of systemic VTE is increased by a number of factors that have already been excellently described by Khorana in his famous score* [2,3], and subsequently reaffirmed by a group of Austrian colleagues who devised and validated an algorithm for simple use[4].
The problem of using a Port-a-Cath for infusion of chemotherapeutic agents should therefore no longer be managed with the sole limited and insufficient perspective of containing the risk of DVT in the upper limb. When the Port-a-Cath is used for the infusion of chemotherapeutics, a risk of both venous and arterial systemic thromboembolism of unpredictable severity is triggered which, at least in subjects at higher risk, makes them deserving of adequate thromboprophylaxis. Obviously this needs to be adjusted carefully in accordance with the progress of the platelet count. We have prophylactic dosages of low pm heparins available. Soon we will know (the CASSINI and AVERT studies are at an advanced stage) if low doses of the new oral anticoagulants can achieve the same result with greater usefulness and lower costs and disruption.
References
References
- Decousus H, Bourmaud A, Fournel P, Bertoletti L, Labruyère C, Presles E, Merah A, Laporte S, Stefani L, Del Piano F, Jacquin JP, Meyer G, Chauvin F. Cancer-associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood 2018;132:707-16.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. evelopment and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902-7.
- Patell R, Rybicki L, McCrae KR, Khorana AA. Predicting risk of venous thromboembolism in hospitalized cancer patients: Utility of a risk assessment tool. Am J Hematol 2017;92:501-7.
- Pabinger I, van Es N, Heinze G, Posch F, Riedl J, Reitter EM, Di Nisio M, Cesarman-Maus G, Kraaijpoel N, Zielinski CC, Büller HR, Ay C. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol 2018;5:e289-98.
* Score of 2 Khorana points for cancers of the stomach or pancreas; 1 point each for the following: cancers of the lung, bladder, testicle, gynaecological cancer or lymphoma; basal platelet count> 350,000; Hb <10 g / dl or use of erythropoietin; leukocyte count> 11,000; BMI> 35. Low thromboembolic risk: score 0; intermediate: 1-2; high:> 3.
