Venous thromboembolism (VTE) is a recurrent risk factor in cancer patients. In particular, patients with hematologic malignancies often experience additional thrombocytopenia as a consequence of the bone marrow infiltration at the disease onset or because of the myelotoxic effects of cancer therapies.
The complex hematological scenario of these patients requires a well-defined strategy to cope with VTE complications and, at the same time, an intervention to reduce bleeding episodes due to concomitant thrombocytopenia. To balance bleeding episodes and VTE risk, the restriction of full-dose anticoagulant use in this subset of patients has been suggested (1-2).
Several studies have suggested the use of low-molecular-weight heparin (LMWH) instead of standard anticoagulants, such as anti-vitamin K (VKA) compounds, to treat bleeding episodes in patients with hematologic malignancies (3-5). Interestingly, the use of the direct oral anticoagulants is not yet recommended for VTE in patients with hematologic malignancies, as it has been shown to be associated with major bleeding events (6,7).
One of the major issues that prevents the identification of a generally accepted strategy to treat bleeding risks in this setting is the fact that cancer patients with thrombocytopenia are usually excluded by randomized clinical trials. This happens because the patient’s low platelet (PLT) count (<30–50 × 109/L) is usually an exclusion criterion. In fact, there are limited studies available on the evaluation of the safety of using LMWH in thrombocytopenic patients with hematologic malignancies.
Therefore, suggestions for safe LMWH administration, according to the PLT count, in thrombocytopenic patients suffering from hematologic malignancies and at risk for VTE have been recently published (8).
These suggestions have been developed by 11 hematologists with documented expertise in thrombosis and hemostasis, methodologists and study coordinators, on the basis of a systematic review of the literature.
The group of experts defined the clinical questions concerning the LMWH dosage with a focus on:
- The relevant population (adult patients with hematologic malignancies, VTE, acute no later than 1 month or non-acute, and thrombocytopenia for more than 3 days)
- The management strategies (administration of different doses of LMWH according to different cut-off values for PLT)
- The outcomes (rates of VTE, bleedings, thrombocytopenia and death)
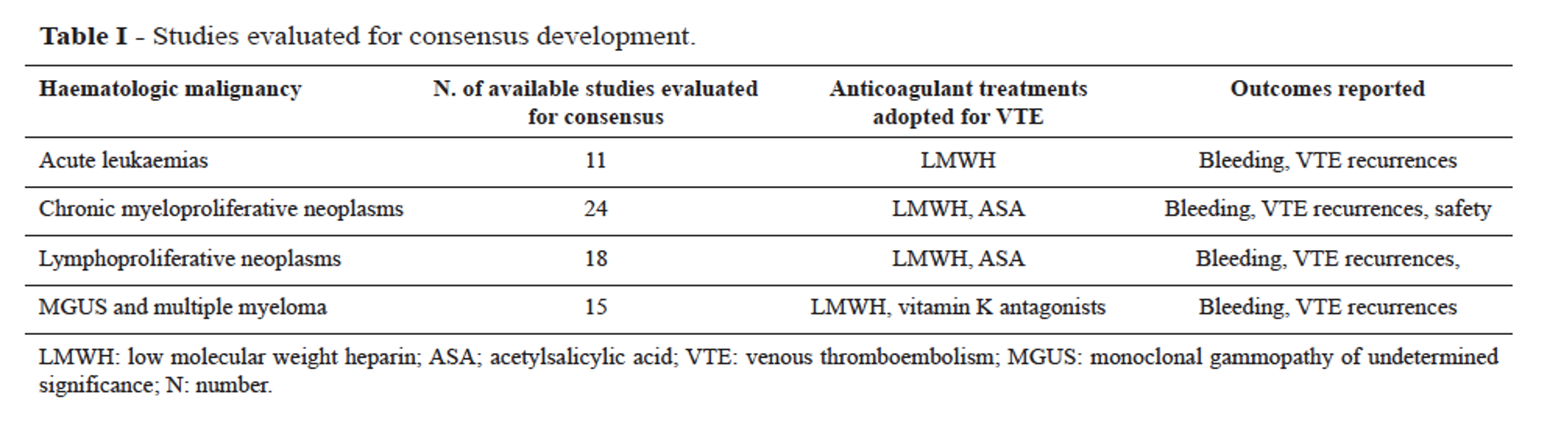
For inclusion, studies had to focus on the therapeutic management of VTE in hematologic cancer patients (Figure 1). Studies on patients with catheter-related thrombosis were also reported and were evaluated separately.To produce the final formal consensus and clinical recommendations, the RAND/UCLA Appropriateness Method was followed (9). A total of 69 studies fulfilled the inclusion criteria. On this basis, the experts debated questions focused on the clinical issues and the management of cancer patients with VTE in the thrombocytopenia setting.
The main recommendations can be summarized as follows:
Acute VTE:
- Therapeutic dose of LMWH for PLT ≥50<100 × 109/L or over
- 50% dose reduction of LMWH for PLT ≥30<50 × 109/L
- Insertion of an inferior vena cava filter, prophylactic dose of LMWH and PLT transfusion for PLT <30 × 109/L
Non-acute VTE:
- Therapeutic dose of LMWH for PLT ≥50<100 × 109/L
- Reduced dose to 50% of LMWH for PLT ≥30<50 × 109/L
The discontinuation of full or reduced therapeutic dose of LMWH is recommended for PLT <30 × 109/L, both in acute and non-acute VTE. For non-acute, catheter-related VTE, the panel agrees on the administration of a therapeutic dose of LMWH for PLT ≥50<100 × 109/L and discontinuation of therapy for PLT <30 × 109/L.
To assess the most appropriate treatment, the panel of experts agreed on the importance of an accurate PLT count monitoring. Repeated PLT transfusions in patients suffering from hematologic malignancies could lead to an immune reaction from PLT infusion with possible adverse events that may compromise the efficacy of the intervention. Therefore, individual cases should be carefully evaluated to define appropriate anticoagulation treatment.
The best practice for the management of VTE recurrence in cancer patients with thrombocytopenia is a matter that will need further assessment by specifically designed studies. Nevertheless, this consensus is the first available report on the establishment of the safe PLT cut-off for LMWH administration in patients with hematologic malignancies with acute and non-acute VTE and may offer a practical guide in clinical settings.
References
- Houghton DE, Key NS, Zakai NA, Laux JP, Shea TC, Moll S. Analysis of anticoagulation strategies for venous thromboembolism during severe thrombocytopenia in patients with hematologic malignancies: a retrospective cohort. Leuk Lymphoma 2017;58(11):2573–2581.
- Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer 2007;110(5):1149–1161.
- Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006;119(12):1062–1072.
- Deitcher SR, Kessler CM, Merli G, et al. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost 2006;12(4):389–396.
- Louzada ML, Majeed H, Wells PS. Efficacy of low-molecular-weight-heparin versus vitamin K antagonists for long term treatment of cancer-associated venous thromboembolism in adults: a systematic review of randomized controlled trials. Thromb Res 2009;123(6):837–844.
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018;378(7):615–624.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018;36(20):2017–2023.
- Napolitano M, Saccullo G, Marietta M, et al. Platelet cut-off for anticoagulant therapy in thrombocytopenic patients with blood cancer and venous thromboembolism: an expert consensus. Blood Transfus 2019;17(3):171–180.
- Fitch KBS, Aguilar MD, Burnand B, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND; 2001.
