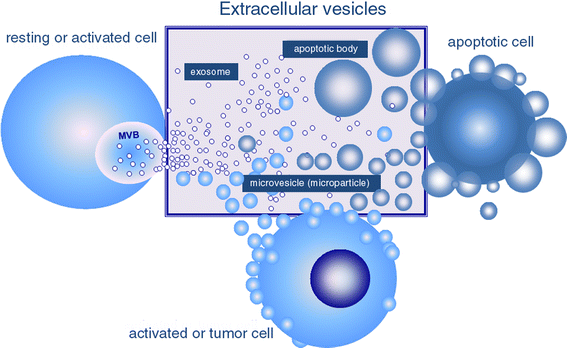
Extracellular vesicles (EV) are submicron vesicles released from activated cells, apoptotic cells and cancer cells (Figure 1) [1].
Pre-analytical variables affect the levels of EVs detected in plasma samples. The most important pre-analytical variable is the method of plasma preparation. Typically, platelet-poor plasma (PPP) is prepared by centrifugation of whole blood at 1,500–2,000 × g for 10–15 minutes at room temperature. Platelet-free plasma (PFP) can be prepared in a variety of ways. However, an International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee (SSC) Collaborative Workshop recommended that PFP should be prepared by centrifugation of whole blood at 2,500 × g for 15 minutes without a brake and then repeat the same cycle to remove platelets.

Several methods are used to analyze EV that measure physical and functional activity of EV. Each method has advantages and disadvantages (Table 1).
Phosphatidylserine-positive (PS+) EV are detected using annexin V by flow cytometry. In addition, cell type-specific markers can be used to determine the cellular origin of the EV. However, the detection limit of conventional flow cytometry is around 200–300 nm and, therefore, flow cytometry cannot accurately measure small EV. Indeed, an ISTH SSC reported that the mean coefficient of variation of platelet-derived EV (CD41+ EV) in well-defined plasma is 66% (range: 64.8–80.3) [3]. Dynamic light scattering can determine the size distribution of EV using Brownian motion but cannot distinguish collapsed or coalesced EV. Currently, nanoparticle tracking analysis is one of the standard methods used to measure EV size and number, but recent studies showed that there is a limitation of accuracy, particularly with small vesicles. Atomic force microscopy (AFM) and electron microscopy (EM) can be used to visualize EV, and EM can also visualize surface proteins using gold labeling. However, these methods are not practical for routine clinical samples. Laser scanning microscopy is an easy method to visualize different antigens on the surface of EV but a major disadvantage is its optical limit (>220 nm) . PS+ EV provide a negatively charged surface that facilitates the assembly of coagulation factors complexes. This is used in functional assays to measure levels of PS+ EV. The ZYMUPHEN™ MP-ACTIVITY assay (Hyphen BioMed) measures PS-dependent thrombin generation whereas the STA-Procoag-PPL assay (Stago) measures PS-dependent clotting time. Levels of tissue factor-positive (TF+) EV can be measured using a two-stage factor Xa generation assay. Two studies have reported detection of TF antigen on EV by conventional flow cytometry and an impedance-based assay [4,5]. However, there was no association between antigen-based assays and EV TF activity raising doubts about the data [6].
Is there an association between PS+ EV and venous thromboembolism (VTE) in cancer patients? As noted above, a major challenge is that there are high levels of PS+ EV in the circulation of healthy individuals. Tesselaar and colleagues found that levels of PS+ EV, measured by flow cytometry, were not different between various types of cancer patients with and without VTE [7]. In contrast, Sartori and colleagues found that levels of PS+ EV, measured using the ZYMUPHEN™ MP-ACTIVITY assay, were elevated in 7 of 11 glioblastoma multiforme (GBM) patients with VTE [8]. However, one study reported a large variation in the ZYMUPHEN™ MP-ACTIVITY assay with high coefficient of variation (40%) in healthy controls [9]
Healthy individuals contain low or undetectable levels of TF+ EV. Therefore, elevated levels of these EV are more easily detected in disease states. We and others have measured levels of EV TF activity in many different cancer patients [10-15]. EV TF activity is elevated in patients with different cancer types, such as pancreatic, gastric, colorectal, lung, breast and ovarian, compared with healthy controls [10,11,14]. However, in single time point studies and longitudinal studies EV TF activity is associated with VTE in pancreatic cancer patients but not in gastric, colorectal, lung and ovarian cancer [12-15]. These data indicate that EV TF activity is a good biomarker of VTE in pancreatic cancer patients.
In conclusion, EV are a new biomarker of diseases but they are difficult to quantify using current methodologies. Functional assays are more sensitive than antigen-based assays. We and others have shown that EV TF activity but not PS+ EV could be used as a biomarker for predicting VTE in some types of cancer.
References
[1] Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011;68(16):2667-2688.
[2] Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res 2017;120(10):1632-1648.
[3] Lacroix R, Judicone C, Mooberry M, et al.; The ISTH SSC Workshop. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013 Apr 2. doi: 10.1111/jth.12207. [Epub ahead of print].
[4] Sartori MT, Della Puppa A, Ballin A, et al. Circulating microparticles of glial origin and tissue factor bearing in high-grade glioma: a potential prothrombotic role. Thromb Haemost 2013;110(2):378-385.
[5] Zwicker JI, Liebman HA, Neuberg D, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res 2009;15(22):6830-6840.
[6] Tatsumi K, Antoniak S, Monroe DM, Khorana AA 3rd, Khorana AA, Mackman N; Subcommittee on Hemostasis and Malignancy of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Evaluation of a new commercial assay to measure microparticle tissue factor activity in plasma: communication from the SSC of the ISTH. J Thromb Haemost 2014;12(11):1932-1934.
[7] Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S, Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost 2009;7(8):1421-1423.
[8] Sartori MT, Della Puppa A, Ballin A, et al. Prothrombotic state in glioblastoma multiforme: an evaluation of the procoagulant activity of circulating microparticles. J Neurooncol 2011;104(1):225-231.
[9] Sorensen D. Assessment of procoagulant activity of microparticles. Aalborg University, Denmark, 51 (2012).
[10] Woei AJFJ, Tesselaar ME, Garcia Rodriguez P, Romijn FP, Bertina RM, Osanto S. Tissue factor-bearing microparticles and CA19.9: two players in pancreatic cancer-associated thrombosis? Br J Cancer 2016;115(3):332-338.
[11] Hisada Y, Thalin C, Lundstrom S, Wallen H, Mackman N. Comparison of microvesicle tissue factor activity in non-cancer severely ill patients and cancer patients, Thromb Res 2018;165:1-5.
[12] Cohen JG, Prendergast E, Geddings JE, et al. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol 2017;146(1):146-152.
[13] Gezelius E, Flou Kristensen A, Bendahl PO, et al. Coagulation biomarkers and prediction of venous thromboembolism and survival in small cell lung cancer: A sub-study of RASTEN – A randomized trial with low molecular weight heparin. PLoS One 2018;13(11):e0207387.
[14] Hisada Y, Alexander W, Kasthuri R, et al. Measurement of microparticle tissue factor activity in clinical samples: A summary of two tissue factor-dependent FXa generation assays. Thromb Res 2016;139:90-97.
[15] Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol 2016;132(6):917-930.
