What is the physician perception and common practice in treatment of venous thromboembolism (VTE) in cancer patients? How did these aspects change through the years? These and other facts are explored in the FRONTLINE (Fundamental Research in Oncology and Thrombosis) survey, and the second round has recently been published.1 Just like the FRONTLINE in 20012, the FRONTLINE 2 survey was developed by the Thrombosis Research Institute in London, UK3 with the contribution of a steering committee of clinician experts in the field of VTE. They collected the responses of 5,233 healthcare professionals in the field of oncology including surgeons, radiotherapists, hematologists, pediatric oncologists, and clinical nurse specialists. The questions of the survey were the same as the FRONTLINE survey and were divided into different sections covering:
- Demographics of the patients;
- Management of VTE in surgical cancer patients;
- Management of VTE in non-surgical cancer patients;
- Thrombosis associated with vascular access devices; and
- Incidental thrombosis in cancer patients.
Results from this kind of survey are very informative as they present a worldwide idea of the perception and clinical practice of physicians who deal with cancer-associated thrombosis issues on a daily basis. Therefore, despite the lack of formal statistical analysis, such data provide a view of the real-world management of patients.
The geographical distribution of the respondents was different in FRONTLINE compared to FRONTLINE 2: in the first survey, the majority of responders were located in western Europe, while in the FRONTLINE 2 survey there was a great increase in participants from North and South America and Asia, with participants mostly coming from Middle Eastern nations. Another difference between the two surveys is the presence of roughly 20% of private practitioners in the FRONTLINE 2, and these were not present in FRONTLINE (Figure 1).1
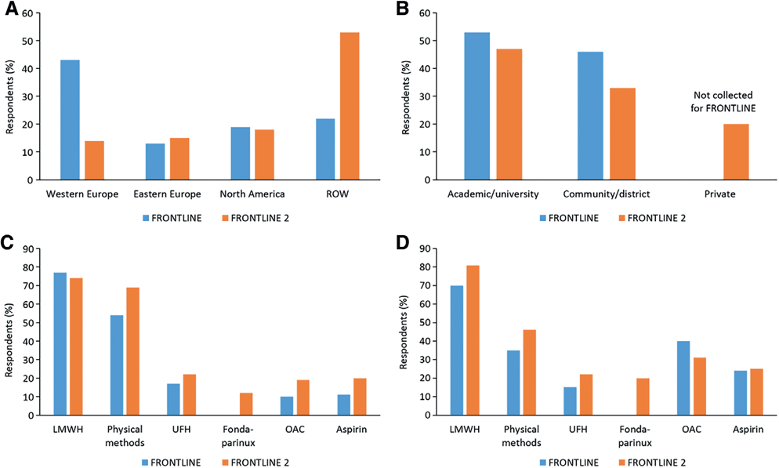
Patients had a variety of cancer types including breast, lung, colon, lymphoma, prostate, and hematologic malignancies. The results of the survey can be summarized as follows:
Results from the survey can be summarized as follows:
- Thrombosis risk in cancer patients is perceived in a proportion of less than one out of five patients, with a slightly higher increase in patients suffering of brain, pancreas and lung cancer;
- With the exception of cancer in children and adults with leukemia, half of the physicians routinely administered VTE prophylaxis, and the reasons for surgically treated patients, were prior history of VTE (74.6%), thrombophilia/thrombocytosis (62.0%) and obesity (59.5%);
- The prophylaxis suggested is mainly low-molecular-weight heparin and unfractionated heparin, with aspirin used more commonly than oral anticoagulants such as warfarin and direct oral anticoagulants [DOACs];
- The prophylaxis period lasts 1 month postoperatively, in general;
- The diagnosis of VTE is based on clinical judgment plus standard imaging;
- A central venous catheter is indicated as a major risk for thrombosis although half of the physicians reported that they rarely give prophylaxis against deep vein thrombosis in patients with a central catheter.
Concerning the use of anticoagulants in prophylaxis, the FRONTLINE 2 survey shows an increment in the use of newer drugs such as fondaparinux and the DOACs, dabigatran and rivaroxaban, which were approved after FRONTLINE. The treatment choice for cancer patients reported in the survey is made individually in day-to-day clinical practice, thus showing what clinicians do on the basis of their perceptions and patients’ preference and feedback, despite the recommendations for VTE prophylaxis in cancer are not different from the ones for patients without cancer. This survey, thus, represents how the general recommendations and guidelines for thrombosis prophylaxis are applied in the real-world setting.
Despite all the limitations of a survey, these results draw attention to the innovations in VTE prophylaxis in cancer patients worldwide and contribute to increasing physicians’ awareness of thrombosis risk in cancer.
References
- Kakkar AK, Bauersachs R, Falanga A, et al. Fundamental Research in Oncology and Thrombosis 2 (FRONTLINE 2): a follow-up survey. Oncologist. 2020 doi:10.1634/theoncologist.2019-0676 (Epub ahead of press).
- Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist. 2003;8(4):381-388. doi:10.1634/theoncologist.8-4-381
- https://www.tri-london.ac.uk/
