Tzu-Fei Wang1, Marc Carrier1*
1Department of Medicine, University of Ottawa at the Ottawa Hospital and The Ottawa Hospital Research Institute, Ottawa, ON, Canada
1. Introduction
Venous thromboembolism (VTE) is a common complication in patients with cancer. Up to 30% of patients will develop VTE during their course of cancer diagnosis and treatment [1]. While anticoagulation is the standard of care for cancer-associated thrombosis, patients with cancer have a significantly increased risk of recurrent VTE despite anticoagulation and bleeding complications on anticoagulation [2,3]. The risk of recurrent VTE is especially high if anticoagulation is discontinued while the cancer remains active [4]. Therefore, major international clinical guidelines have recommended to continue anticoagulation for an extended duration as long as cancer is active and/or there are ongoing anticancer therapies [5-7]. However, previous randomized controlled trials (RCTs) assessing the acute treatment of cancer-associated thrombosis mostly limited follow-up duration to 6 months [8-11], so data on the treatment and outcomes beyond the initial 6 months were lacking. In the recent years, this has been identified as an important knowledge gap and more clinical studies and RCTs were specifically designed to evaluate extended treatment duration in patients with cancer-associated thrombosis (i.e., beyond the initial 6 months) [12,13]. In this article, we aim to review data from pertinent clinical studies, focusing on extended duration of anticoagulation and associated outcomes. Table 1 (cohort studies) and Table 2 (RCTs) summarize the studies discussed.
Table 1. Summary of outcomes in cohort studies evaluating extended duration of anticoagulation for cancer-associated thrombosis (after the initial 3-6 months) 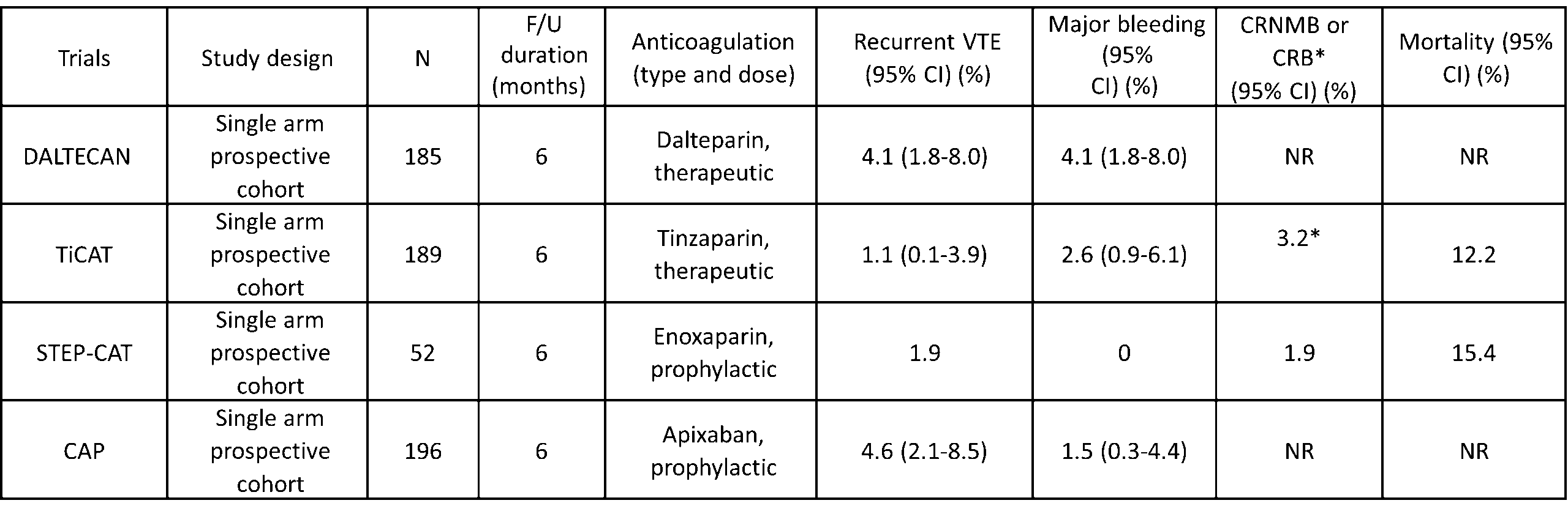 Abbreviations: CI – confidence interval; CRNMB – clinically relevant non-major bleeding; CRB – clinically relevant bleeding (major bleeding and CRNMB); F/U – follow up; NR- not reported; VTE – venous thromboembolism.
Abbreviations: CI – confidence interval; CRNMB – clinically relevant non-major bleeding; CRB – clinically relevant bleeding (major bleeding and CRNMB); F/U – follow up; NR- not reported; VTE – venous thromboembolism.
Table 2. Summary of outcomes in randomized controlled trials evaluating extended duration of anticoagulation for cancerassociated thrombosis (after the initial 6 months) 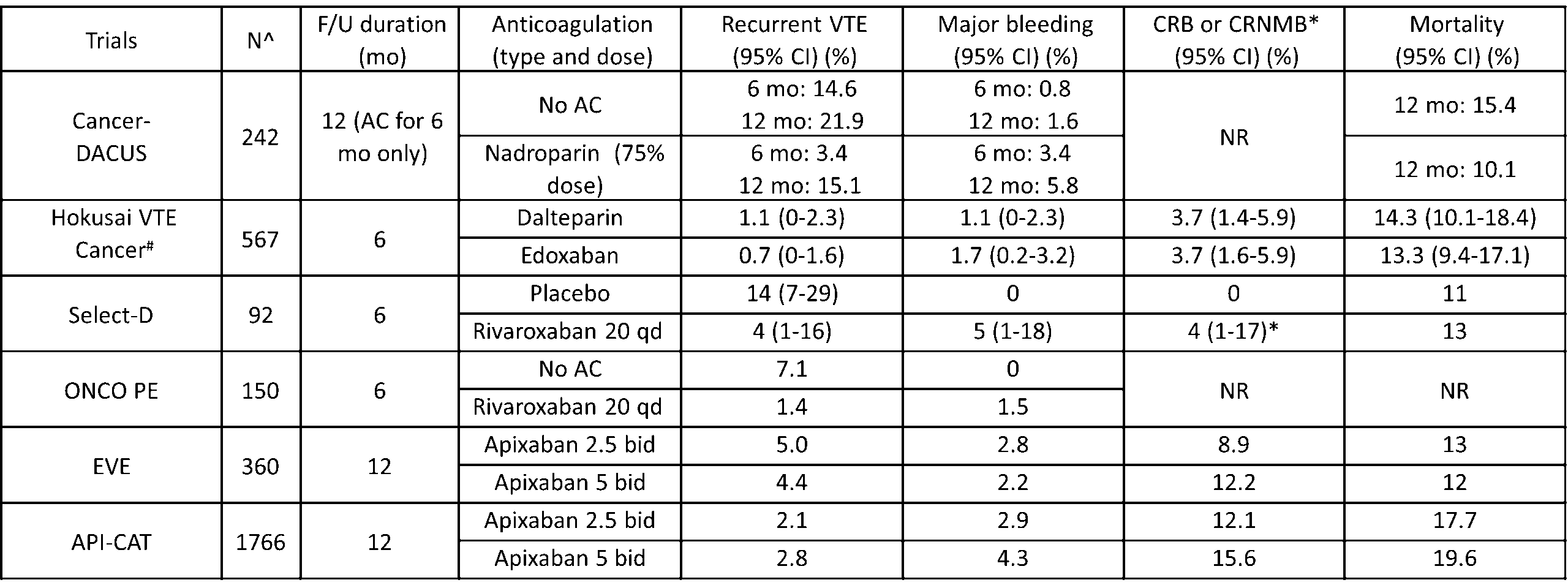 ^Number of patients are at the time of 6 months #Rates are during treatment with study drug
^Number of patients are at the time of 6 months #Rates are during treatment with study drug
Abbreviations: AC – anticoagulant; bid – twice daily; CI – confidence interval; CRNMB – clinically relevant non-major bleeding; CRB – clinically relevant bleeding (major bleeding and CRNMB); F/U – follow up; mo – months; NR- not reported; qd – daily; VTE – venous thromboembolism.
2. Low-molecular-weight heparin for extended anticoagulation
The practice-changing CLOT trial showed that low-molecular-weight heparin (LMWH) was superior in the reduction of recurrent VTE compared to vitamin K antagonists without an increased risk of bleeding in patients with cancer-associated thrombosis [8]. Hence, LMWH became the standard of care for cancer-associated thrombosis for the past two decades. Therefore, initial studies evaluating extended treatment duration mainly used LMWH. The Cancer-DACUS study included 347 patients with cancer-associated proximal lower extremity deep vein thrombosis (DVT) after the initial 6 months of anticoagulation [14]. A lower extremity doppler ultrasound was done following 6 months of treatment, and anticoagulation was discontinued in patients without residual vein obstruction (N=105). The remaining 242 patients with residual vein obstruction on lower extremity ultrasound were randomized to discontinue anticoagulation or continue intermediate dosing of LMWH (75% dose of nadroparin) for an additional 6 months. The 12-month cumulative incidences of recurrence VTE were 21.9% and 15.1% in patients who had discontinued or continued anticoagulation, respectively (Hazard Ratio [HR] 1.58, 95% confidence interval [CI] 0.8-3.0). The 12month cumulative incidences of major bleeding were 1.6% in patients who had discontinued anticoagulation and 5.8% in those receiving intermediate dosing of LMWH. The 12-month incidences of recurrent VTE and major bleeding in those without residual vein obstruction (in whom anticoagulation was stopped) were 2.8% and 1.9%, respectively. This study showed that the risks of recurrent VTE were significantly higher in patients with residual vein obstruction regardless of whether anticoagulation was continued or not.
Next came the DALTECAN study, a single arm, prospective cohort study where all patients with cancer-associated thrombosis were treated with standard dalteparin regimen (200 units/kg daily for one month, followed by 150 units/kg daily) for up to 12 months [15]. Among 334 patients enrolled, 185 (55.4%) continued the study beyond 6 months. The 6-month cumulative incidences of recurrent VTE and major bleeding events after the initial 6 months (i.e., months 7-12) were both 4.1% (95% CI 1.8-8.0), lower than the incidences in the first 6 months. Subsequently, the TiCAT study, another single arm prospective cohort study in patients with cancer-associated thrombosis treated on therapeutic dose of Tinzaparin (175 units/kg daily) for 12 months was reported [16]. A total of 247 patients were enrolled, of whom 189 continued beyond 6 months. The cumulative incidences of recurrent VTE and clinically relevant bleeding events (composite major and clinically relevant non-major bleeding [CRNMB]) were 1.1% (95% CI 0.1-3.9) and 3.2% in months 7-12, respectively. The 7 to 12-month incidences were also lower than the incidences in the first 6 months.
More recently, the STEP-CAT study was designed to evaluate a “step-down” (prophylactic) dose of LMWH as the extended anticoagulation regimen [17]. Fifty-two patients with a first cancer-associated VTE were enrolled after at least 3 months of therapeutic anticoagulation. After enrollment, patients received enoxaparin 40 mg daily (prophylactic dose) for up to 26 weeks. The study was stopped prior to achieving planned enrollment (N=150) given poor recruitment. During the mean (standard deviation) follow up of 5.6 (1.4) months, one recurrent VTE (incidental pulmonary embolism [PE]) (1.9%) was diagnosed. No major bleeding, one (1.9%) CRNMB and one (1.9%) minor bleeding episode were also observed. Although the study was terminated early with a modest sample size, the low event rates suggested that reduced dose LMWH may be safe and effective for the extended treatment duration for patients with cancer-associated thrombosis, however, more data are needed.
3. Direct oral anticoagulants (DOACs) for extended anticoagulation
The Hokusai VTE Cancer trial was the first major RCT in cancer-associated thrombosis that followed patients beyond 6 months after the diagnosis of VTE [18]. The trial randomized 1046 patients with acute cancer-associated thrombosis to dalteparin or edoxaban for a total duration of 12 months. Among them, 567 continued anticoagulation beyond 6 months. The rates of recurrent VTE between 6 and 12 months on therapeutic dose with either edoxaban or dalteparin were low, 0.7% and 1.1%, respectively. Clinically relevant bleeding (major and CRNMB) event rates were also low and occurred in 3.7% of patients on either anticoagulant.
The Select-D trial randomized 406 patients with acute cancer-associated thrombosis to dalteparin or rivaroxaban for a total of 6 months [19]. After the initial 6 months of anticoagulation, a lower extremity doppler ultrasound was obtained to determine the presence of residual vein obstruction. Patients with index PE or residual vein obstruction on ultrasound underwent a second randomization to rivaroxaban 20 mg daily or placebo for an additional 6 months. As in the Cancer-DACUS study, anticoagulation was discontinued in patients without residual vein obstruction [14]. The study was terminated early given slow recruitment, with a total of 92 patients undergoing second randomization. A reduced risk of recurrent VTE was reported in those receiving rivaroxaban compared to placebo (4% vs. 14%, HR 0.32; 95% CI 0.06-1.5). Additionally, among the 35 patients without residual vein obstruction on ultrasound at 6 months, 29 of whom discontinued anticoagulation per protocol, and none developed recurrent VTE between 6 and 12 months. This study suggested again that the lack of residual vein obstruction may be associated with a lower risk of recurrent VTE.
The CAP study was a prospective, single arm cohort study in Norway, where 296 patients with cancer-associated thrombosis were treated with therapeutic dose of apixaban (5 mg twice daily) [20]. After the initial 6 months, 196 continued the study and received a reduced dose of apixaban (2.5 mg twice daily) for an additional 30 months.
The risks of recurrent VTE between 6 and 12 months were similar to the initial 6 months (4.6% vs. 4.0%) while the risks of major bleeding were lower (1.5% vs. 5.4%). The investigators concluded that the reduced dose of apixaban after the initial 6 months of therapeutic dose may be a reasonably safe regimen.
The ONCO PE trial was a Japanese multi-center, open-labelled RCT comparing treatment with therapeutic dose of rivaroxaban for 18 months to 6 months in patients with cancer and acute low-risk PE [21]. The study was stopped early for slow recruitment due to COVID-19 pandemic and a total of 179 patients were enrolled. The 18-month treatment duration was superior to the 6-month duration in the reduction of recurrent VTE without an increased risk of major bleeding. Between 6 and 12 months, the cumulative incidence of recurrent VTE was significantly lower in patients continuing therapeutic dose of rivaroxaban (1.4%) compared to those discontinuing anticoagulation (7.1%), and the incidences of major bleeding were similar at 1.5% and 0%, respectively.
A systematic review summarized the evidence on the rates of recurrent VTE and major bleeding beyond 6 months in patients with cancer-associated thrombosis [22]. A total of 11 studies including 3019 patients were included. Rates of recurrent VTE between 6 and 12 months varied significantly (1.1-12.0%) depending on the use of anticoagulation and/or the presence of residual vein obstruction, with the highest risk of recurrent VTE found in patients with residual vein obstruction not receiving anticoagulation. Rates of major bleeding between 6 and 12 months were relatively low at 2 to 5%. Both risks of recurrent VTE and bleeding events were lower beyond 6 months compared to those in the first 6 months after index VTE.
4. RCTs evaluating extended anticoagulation
Most recently, two RCTs were specifically designed to evaluate the optimal dose of DOAC for extended treatment duration in patients with cancer-associated thrombosis. The EVE trial is a multi-center RCT in the United States, comparing a reduced dose of apixaban (2.5 mg twice daily) to full dose (5 mg twice daily) for 12 months in patients who had completed 6-12 months of anticoagulation for cancer-associated thrombosis [12]. In addition to the traditional PE and lower extremity DVT, the investigators included upper extremity DVT and unusual site VTE (such as cerebral or splanchnic vein thromboses), accounting for 10.3% and 6.7% of the study population, respectively.
Among the 360 patients included in the analysis, the 12-month cumulative incidences of the primary outcome, clinically relevant bleeding (major bleeding and CRNMB) events, were 8.9% and 12.2% in patients receiving reduced and full dose of apixaban, respectively (HR 0.72; 95% CI 0.38-1.37). The risk of recurrence VTE was no different (5.0% vs. 4.4%, HR 1.00; 95% CI 0.40-2.53) between the two groups. While this study provided promising data, an important concern was insufficient sample size resulting in an underpowered study.
The results of the API-CAT trial were recently reported [13]. The API-CAT trial is an international, double-blind RCT, where 1766 patients with proximal lower extremity DVT and/or PE were enrolled after at least 6 months of anticoagulation and randomized to a reduced dose of apixaban (2.5 mg twice daily) or full dose (5 mg twice daily) for 12 months. The cumulative incidences of recurrent VTE in patients receiving the reduced and full doses of apixaban were 2.1% and 2.8%, respectively, achieving non-inferiority, while the cumulative incidence of clinically relevant bleeding events (major bleeding and CRNMB) was significantly lower in patients receiving a reduced dose of apixaban compared to full dose (12.1% vs. 15.6%; HR 0.75; 95% CI, 0.58 – 0.97). This study showed that in patients with cancer-associated thrombosis requiring anticoagulation for an extended duration, a reduced dose of apixaban (2.5 mg twice daily) likely provides the optimal benefit and risk consideration and can change practice.
We performed a pooled analysis of the EVE and API-CAT trials to summarize the results by using the Review Manger (RevMan), version 8.21.0, according to the recommendations from the Cochrane Collaboration (Figure 1) [23]. The risk ratios (RR) and 95% CIs were calculated using random effect models. When the results of the two trials were pooled, there were no statistical differences in the risk of recurrent VTE (RR 0.87, 95% CI 0.53-1.45) or major bleeding events (RR 0.73, 95% CI 0.47-1.17) between the reduced (2.5 mg twice daily) and full doses (5 mg twice daily) of apixaban (Figure 1A and 1B). However, the reduced dose of apixaban was associated with a significantly lower risk of clinically relevant bleeding (major and CRNMB) events compared to the full dose (RR 0.77, 95% CI 0.62-0.97) (Figure 1C).
Figure 1. Pooled analysis of the EVE and APT-CAT trials
A) Recurrent venous thromboembolism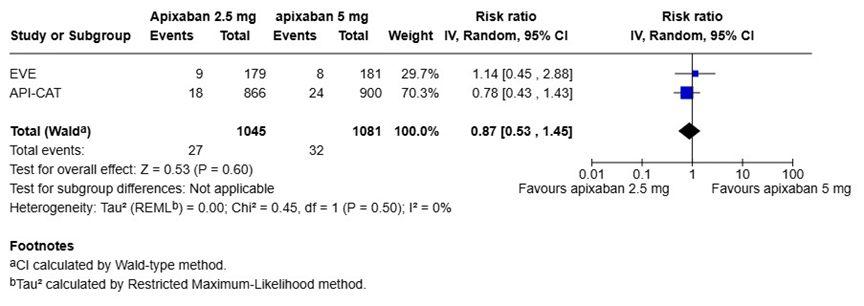
B) Major bleeding events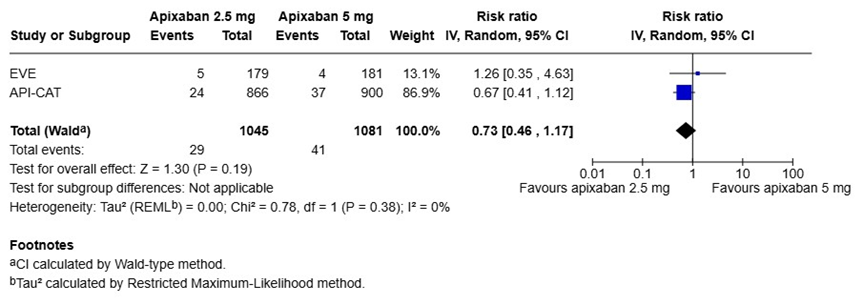
C) Clinically relevant bleeding events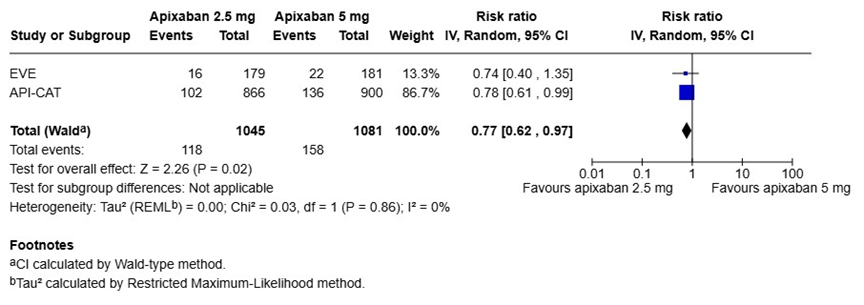
In recent years, with continued advances in oncological treatments such as immune checkpoint inhibitors and many other targeted therapies, patients have significantly improved survival, but at the same time, the durations when they are at risk for VTE are also prolonged. Therefore, the optimal extended treatment to balance the risk of bleeding with the benefit of reducing recurrent VTE is increasingly important. Based on the available evidence, extended treatment duration with reduced dose anticoagulation showed superior safety profile compared to full dose anticoagulation in this patient population.
5. Conclusions
In patients with cancer-associated thrombosis, the risks of recurrent VTE or bleeding outcomes are generally lower after the initial 6 months as compared to the initial 6 months. Nonetheless, the risks remain elevated especially in the presence of active cancer and/or ongoing cancer therapies, and continuing anticoagulation is recommended. Recent RCTs showed that anticoagulant dose can be safety reduced for extended treatment duration to optimize the risk and benefit consideration.
Acknowledgement
M. Carrier is the recipient of Clinical Research Chair from the University of Ottawa in Cancer and Thrombosis.
Conflict of interest
T-F. Wang declares no conflict of interest.
M. Carrier reports grants from Pfizer, personal fees from BMS, Leo Pharma, Bayer, Pfizer, Anthos, Regeneron and Sanofi.
Correspondence:
Tzu-Fei Wang, MD, MPH
501 Smyth Road, Box 201
Ottawa, ON K1H 8L6, Canada
Tel: +1 613-737-8899
Email: tzwang@toh.ca
Keywords:
cancer; thromboembolism; anticoagulation; extended duration.
References
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-21.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484-3488.
- Ainle FN, Kevane B. Which patients are at high risk of recurrent venous thromboembolism (deep vein thrombosis and pulmonary embolism)? Blood Adv. 2020;4(21):5595-5606.
- Barca-Hernando M, Lopez-Ruz S, Marin-Romero S, et al. Risk of recurrent cancerassociated thrombosis after discontinuation of anticoagulant therapy. Res Pract Thromb Haemost. 2023;7(2):100115.
- Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5(4):927-974.
- Key NS, Khorana AA, Kuderer NM, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38(5):496-520.
- NCCN guidelines in Cancer-Associated Venous Thromboembolic Disease, Version 1.2025, NCCN guideline panel; 2025.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA. 2015;314(7):677-686.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol. 2018;36(20):2017-2023.
- Agnelli G, Becattini C, Meyer G, et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med. 2020;382(17):1599-1607.
- McBane RD, 2nd, Loprinzi CL, Zemla T, et al. Extending venous thromboembolism secondary prevention with apixaban in cancer patients. The EVE trial. J Thromb Haemost. 2024;22(6):1704-1714.
- Mahe I, Carrier M, Mayeur D, et al. Extended Reduced-Dose Apixaban for CancerAssociated Venous Thromboembolism. N Engl J Med. 2025.
- Napolitano M, Saccullo G, Malato A, et al. Optimal duration of low molecular weight heparin for the treatment of cancer-related deep vein thrombosis: the Cancer-DACUS Study. J Clin Oncol. 2014;32(32):3607-3612.
- Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost. 2015;13(6):1028-1035.
- Jara-Palomares L, Solier-Lopez A, Elias-Hernandez T, et al. Tinzaparin in cancer associated thrombosis beyond 6 months: TiCAT study. Thrombosis Research. 2017;157:90-96.
- Popov J, Coelho S, Carrier M, et al. Step down to 6 months of prophylactic-dose low molecular weight heparin after initial full-dose anticoagulation for the treatment of cancerassociated thrombosis (STEP-CAT): A pilot study. J Thromb Haemost. 2022;20(8):1868-1874.
- Di Nisio M, van Es N, Carrier M, et al. Extended treatment with edoxaban in cancer patients with venous thromboembolism: A post-hoc analysis of the Hokusai-VTE Cancer study. J Thromb Haemost. 2019;17(11):1866-1874.
- Marshall A, Levine M, Hill C, et al. Treatment of cancer-associated venous thromboembolism: 12-month outcomes of the placebo versus rivaroxaban randomization of the SELECT-D Trial (SELECT-D: 12m). J Thromb Haemost. 2020;18(4):905-915.
- Larsen TL, Garresori H, Brekke J, et al. Low dose apixaban as secondary prophylaxis of venous thromboembolism in cancer patients – 30 months follow-up. J Thromb Haemost. 2022;20(5):1166-1181.
- Yamashita Y, Morimoto T, Muraoka N, et al. Rivaroxaban for 18 Months Versus 6 Months in Patients With Cancer and Acute Low-Risk Pulmonary Embolism: An Open-Label, Multicenter, Randomized Clinical Trial (ONCO PE Trial). Circulation. 2025;151(9):589-600.
- Moik F, Colling M, Mahe I, Jara-Palomares L, Pabinger I, Ay C. Extended anticoagulation treatment for cancer-associated thrombosis-Rates of recurrence and bleeding beyond 6 months: A systematic review. J Thromb Haemost. 2022;20(3):619-634.
- Cocharne Handbook for Systemic Reviews of Interventions. https://training.cochrane.org/handbook.
