Nikola Vladic¹, Cihan Ay¹
¹Division of Hematology and Haemostaseology, Department of Medicine I, Medical University of Vienna, Vienna, Austria
1. High risk of VTE in ambulatory cancer patients, a persisting challenge
Advancements in anti-cancer treatment have significantly improved patient survival over the last decades, transforming many cancers from rapidly fatal diseases into chronic conditions [1]. This shift has increased the prevalence of cancer, as patients live longer due to improved diagnostics and personalized treatment strategies [2,3]. Consequently, the clinical focus has expanded beyond managing the underlying malignancy only to address cancer-related complications and patient quality of life. Venous thromboembolism (VTE) remains a common and particularly burdensome complication of cancer and its treatments adding to the overall burden of disease [4,5].
Cancer-associated VTE affects up to 20% of patients with cancer, occurring mostly within the six months of diagnosis. The risk depends on cancer type, stage and treatment modality [6]. These rates are comparable and in some cohorts surpass the reported 5% six-month rate of VTE in acutely ill hospitalized patients, a population in which thromboprophylaxis is universally accepted and widely practiced [7]. The incidence of VTE in patients with cancer is rising, driven by several factors, including the increasing global cancer burden, improved detection of thrombotic events, and the increasing awareness of cancer-associated VTE [8]. Additionally, prolonged survival has resulted in longer exposure to systemic anti-cancer therapies, many of which increase the risk of VTE while also contributing to heightened risks of arterial thromboembolism (ATE) and bleeding [9–11]. The clinical consequences of cancer-associated VTE extend well beyond the acute thrombotic event itself. Patients who develop a VTE suffer from increased psychological distress, the need for prolonged hospitalization, anti-cancer treatment interruptions and post-thrombotic syndrome [12,13]. Importantly, VTE is also independently associated with a shorter progression free and overall survival rate [14].
To combat the high burden of cancer-associated VTE, several guidelines recommend the use of validated risk assessment models (RAMs) to guide primary thromboprophylaxis decisions in ambulatory patients with cancer [Table 1, 15–19].
Table 1. Overview of current guidelines on prophylactic anticoagulation in patients with cancer.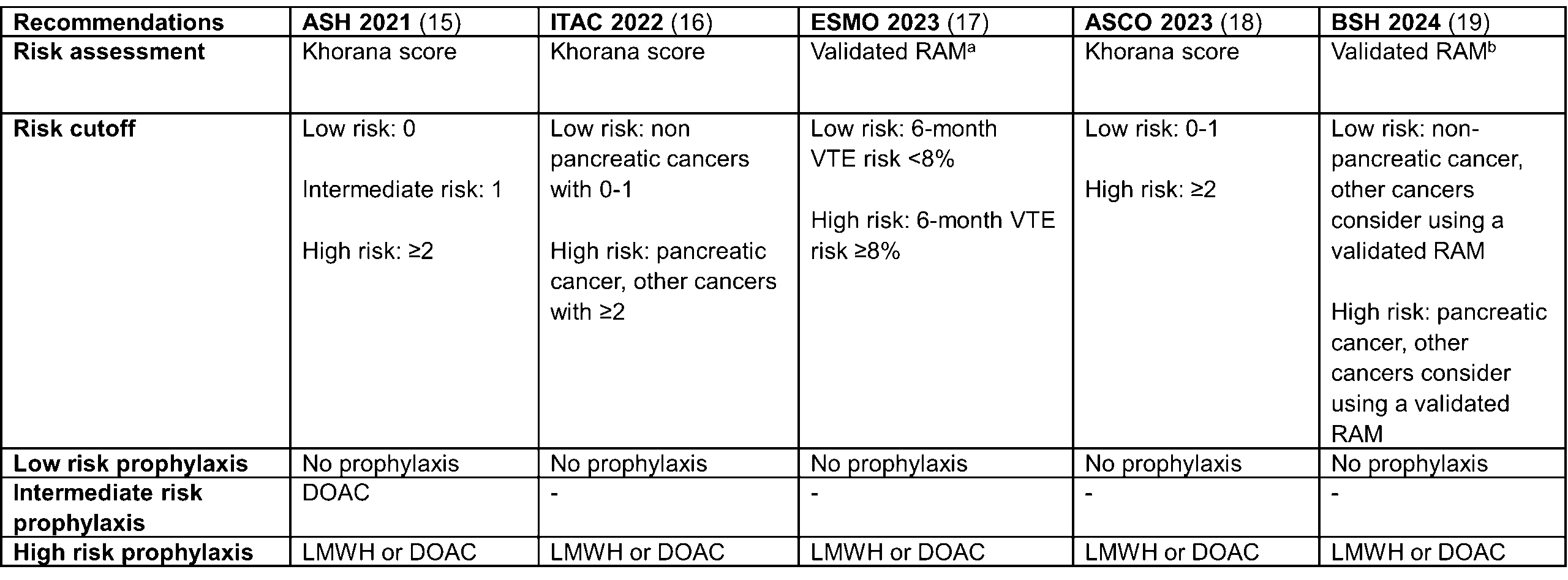 RAM = risk assessment model, LMWH = low molecular weight heparin, DOAC = direct oral anticoagulant
RAM = risk assessment model, LMWH = low molecular weight heparin, DOAC = direct oral anticoagulant
aSuch as the Khorana score, COMPASS-CAT or CATScore
bAny validated RAM
These RAMs were specifically developed to stratify patients according to their thrombotic risk, thereby tailoring VTE prophylaxis to those patients who are most likely to benefit, while at the same time minimizing unnecessary anticoagulation exposure which leads to high bleeding rates [20]. Several randomized controlled trials and meta-analyses have demonstrated the efficacy of risk-based prophylactic anticoagulation in ambulatory patients with cancer, significantly reducing VTE. Notably, these studies confirm a low incidence of bleeding when risk-driven prophylaxis is appropriately applied, and some of the data suggests that prophylactic anticoagulation may carry an overall survival benefit, although these findings remain debated and warrant further investigation [21–23].
Despite guideline recommendations and the proven effectiveness of prophylactic anticoagulation, real-world implementation of routine thromboprophylaxis remains poor [24]. There are several barriers, which lead to poor guideline adherence and these include limited clinician and patient awareness of the thrombotic risk in cancer, fear of bleeding in already frail, elderly and multimorbid patients, issues of polypharmacy and drug-drug interactions as well as difficult to routinely implement RAMs, some of which rely on more than 10 clinical parameters and incorporate hemostatic biomarkers, such as D-dimer [6,25–27]. This requirement poses challenges because elevated D-dimer levels are common among cancer patients but are also widely implemented in several clinical prediction rules for ruling out acute VTE leading to dilemmas regarding the interpretation of results and subsequent diagnostic actions and making physicians hesitant to order D-dimer levels for risk stratification [28,29]. Coupled with the time constraints inherent in modern clinical practice these factors collectively contribute to the inadequate guideline adherence [Figure 1].
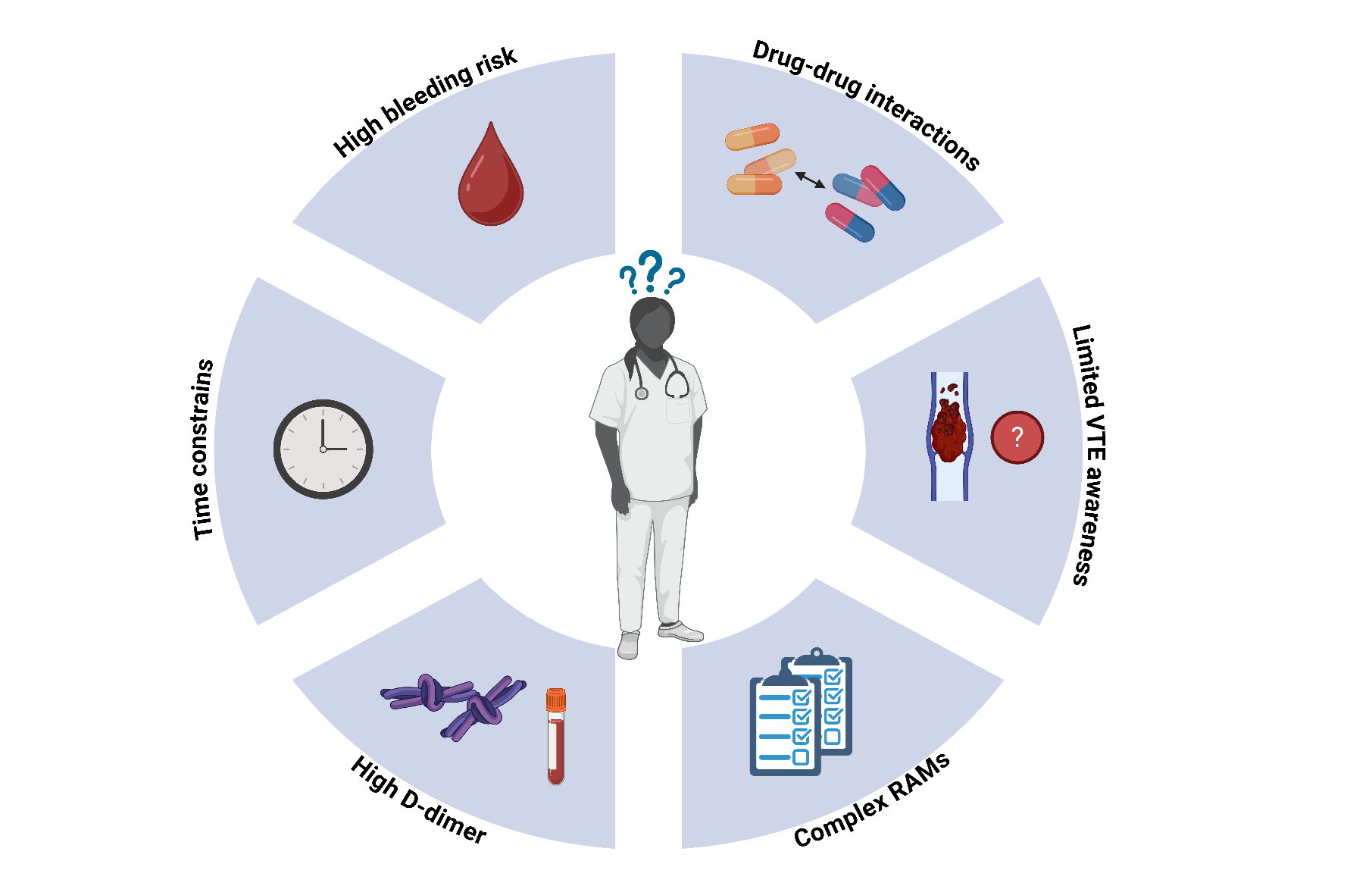 Figure 1. Barriers to the implementation of primary VTE prophylaxis in ambulatory patients with cancer. Overview of common barriers contributing to the poor use of primary venous thromboembolism prophylaxis in ambulatory patients with cancer, including clinician related factors, risk assessment complexities, and practical constraints in clinical settings. RAM = risk assessment model, VTE = venous thromboembolism.
Figure 1. Barriers to the implementation of primary VTE prophylaxis in ambulatory patients with cancer. Overview of common barriers contributing to the poor use of primary venous thromboembolism prophylaxis in ambulatory patients with cancer, including clinician related factors, risk assessment complexities, and practical constraints in clinical settings. RAM = risk assessment model, VTE = venous thromboembolism.
2. Immune checkpoint inhibitor therapy and the risk of VTE
Immune checkpoint inhibitor (ICI) therapy has revolutionized anti-cancer treatment, leading to improved survival in numerous cancer types [30,31]. The use of ICI therapy has been rapidly expanding in the last decades with current estimates that more than 30% of all patients with cancer are eligible for ICIs [32].
ICIs, including anti-PD-1, anti-PD-L1 and anti-CTLA-4 monoclonal antibodies, act by increasing the efficiency of T-cells in eliminating cancer cells [33]. However, this activation of the immune system can also trigger extensive inflammation manifesting as immune-related adverse events [34]. Given the well-established link between inflammation and coagulation ICIs plausibly contribute to an increased thrombotic risk [35]. Several trials have reported high rates of VTE in patients on ICIs, comparable to rates in patients receiving chemotherapy, a well-established risk factor for VTE [36–38]. However, data is heterogeneous as similar studies have reported low rates of VTE associated with ICIs [39,40]. This discordance calls for further investigation. Unfortunately, thrombotic events are rarely adequately reported in clinical trials, leading to an underestimation of the thrombotic risks associated with these therapies at the time of their approval. Consequently, the real-world incidence of thrombotic events related to immunotherapy is identified only after these agents are integrated into routine clinical practice [41]. Potentially even more established than the risk of VTE in patients on ICIs is the high risk of ATE, reported in several studies [42–44] with preclinical research also demonstrating the pro-atherogenic effects of ICIs in animal models [45–47].
Addressing the continuously evolving landscape of oncology treatments will be essential to effectively manage and prevent cancer-associated VTE. Our recent study underscores the limited predictive performance of currently available RAMs in contemporary cancer patient cohorts characterized by widespread use of ICIs and targeted therapies. Historically, these RAMs were developed and validated in populations treated with primarily chemotherapy alone, failing to capture the thrombotic risks associated with modern therapeutic modalities [48].
3. NSCLC and VTE risk, impact of oncogenic drivers and targeted therapies
Patients with non-small cell lung cancer (NSCLC) exhibit varying risks of VTE depending on presence of established risk factors (Figure 2). These risk factors include cancer stage, type of systemic anticancer therapy, prior history of VTE, age and specific genomic alterations within the tumor [6,49].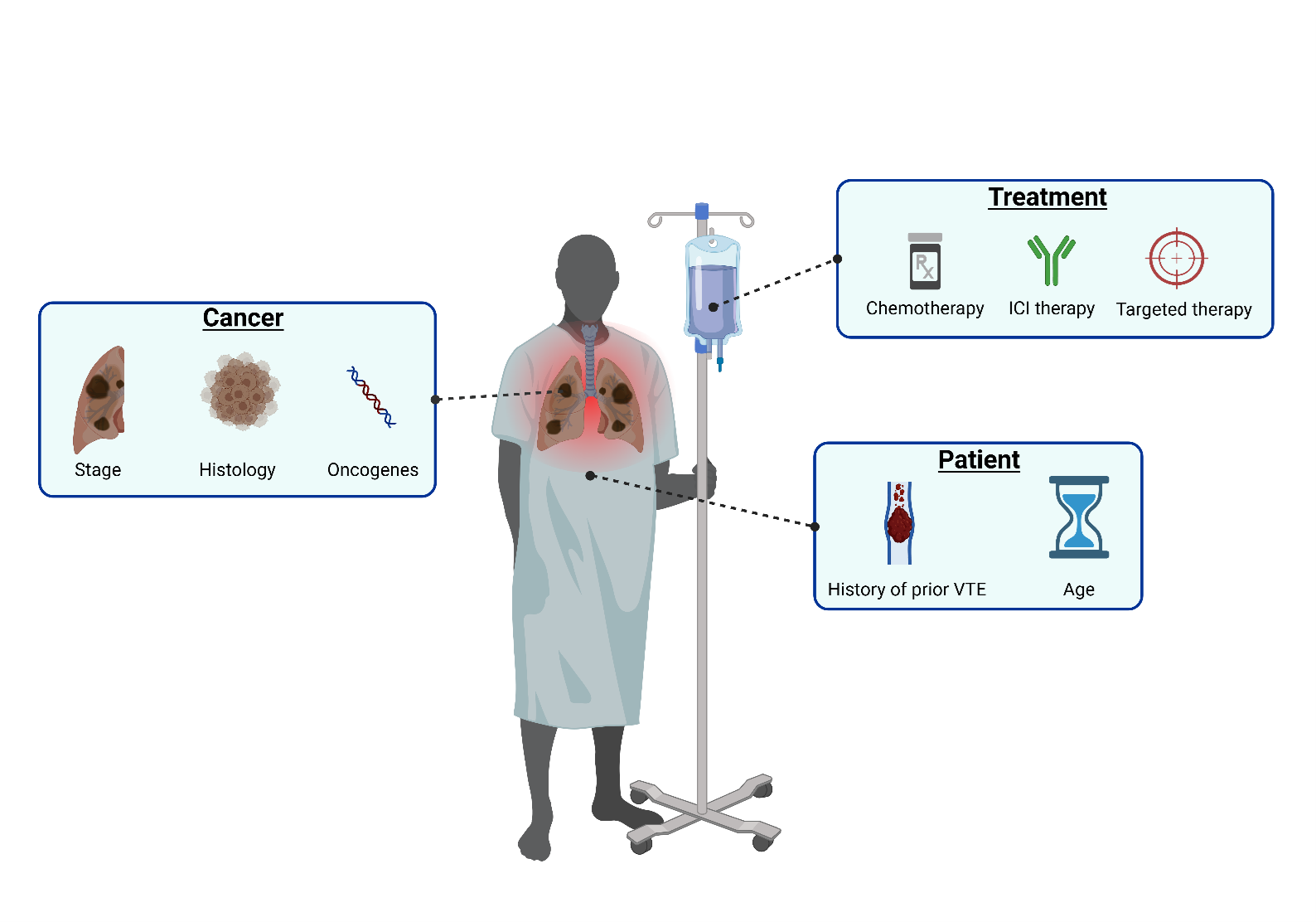 Figure 2. Risk factors for VTE in patients with NSCLC. Overview of major risk factors contributing to the elevated risk of venous thromboembolism in patients with non-small cell lung cancer, categorized into cancer-related, treatment-related, and patient-related risk factors. ICI = immune checkpoint inhibitor.
Figure 2. Risk factors for VTE in patients with NSCLC. Overview of major risk factors contributing to the elevated risk of venous thromboembolism in patients with non-small cell lung cancer, categorized into cancer-related, treatment-related, and patient-related risk factors. ICI = immune checkpoint inhibitor.
Among the oncogenic drivers associated with increased VTE risk in NSCLC, genetic rearrangements in anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) have been consistently associated with high VTE risk [50,51]. Several studies have demonstrated notably higher VTE rates among patients with these rearrangements compared to those with wild-type NSCLC or epidermal growth factor receptor (EGFR) mutations [49]. Data from a meta-analysis demonstrated that the overall incidence of VTE with ALK, ROS1, wild type and EGFR cancers are: 41%, 30%, 14% and 12% respectively [52]. The thrombotic risk in patients with EGFR mutations tends to be lower and comparable to the rates seen in wild-type NSCLC populations. However, it remains critical to recognize that even within EGFR-mutated NSCLC, variation can exist based on the specific type of EGFR mutation with a recent retrospective analysis showing a higher 4-week VTE ratio in patients with a mutation in exon 21 (22.2%) versus a mutation in exon 19 (9.0%) [53]. The impact of systemic anticancer treatments, including chemotherapy, ICIs, and targeted therapies, on VTE risk is also high in patients with NSCLC [54]. Historically, chemotherapy has been recognized as a significant risk factor for VTE, with incidence rates varying widely depending on the chemotherapy regimen employed [55]. Platinum-based chemotherapies, frequently used in advanced NSCLC, have consistently been associated with elevated thrombotic risk [55,56]. In a Danish population-based study of patients with metastatic NSCLC, the 1-year cumulative incidence of VTE varied by type of systemic anti-cancer therapy, ranging from 7.7% in those receiving chemotherapy to 12.2% in those treated with ICIs, and 12.5% in patients receiving targeted therapies [54]. Less data exists on the thrombotic risk associated with specific targeted agents in NSCLC, particularly agents directed against oncogenic drivers such as ALK, ROS1, and EGFR. In a recent meta-analysis crizotinib, a dual ALK and ROS1 inhibitor, has been linked to an increased risk of VTE compared to chemotherapy (OR 0.27, 95% CI 0.10-0.62), brigatinib (OR 0.18; 95% CI 0.04–0.60), and ceritinib (OR 0.10; 95% CI 0.02–0.43). This elevated risk may reflect both the underlying oncogenic pathway and drug-specific effects, warranting close clinical monitoring and further investigation [57].
Notably, data on novel targeted therapies such as amivantamab, a bispecific antibody targeting EGFR and MET pathways, have raised significant concerns due to a high incidence of VTE ranging from 10% but up to 21-37% in combinations with lazertinib [58]. Specifically, in EGFR-mutated NSCLC patients within the MARIPOSA trial, VTE occurred in 37% of patients receiving amivantamab-lazertinib, compared to 9% with osimertinib. Similarly in MARIPOSA-2 the VTE rates were 22% with amivantamab-lazertinib and chemotherapy versus 5-10% in the control arms [49]. Additional trials, including PAPILLON, CHRYSALIS-2, and PALOMA-3, reported VTE rates ranging from 10% to 30%, with highest risks seen in combination regimens and early after treatment initiation. Notably, prophylactic anticoagulation in PALOMA-3 reduced VTE rates substantially, indicating the urgent need for preventative strategies in this very high-risk setting [49].
4. Conclusion
The high risk of VTE in patients with cancer highlights a critical need for effective primary prophylaxis [5,6]. Despite robust evidence and clear guideline recommendations, clinical implementation remains poor due to persistent barriers, including limited awareness, fear of bleeding, drug-drug interactions and complexity of existing RAMs [6,25–27]. Furthermore, the rapid adoption of novel anticancer therapies, such as ICIs and targeted treatments, has introduced new thrombotic risks that current risk stratification tools fail to adequately address [48]. Specifically, ICIs have been reported to elevate thrombotic risk via inflammatory adverse effects, while specific oncogenic drivers in NSCLC (particularly ALK and ROS1 rearrangements) and novel targeted therapies such as Amivantamab have emerged as significant risk factors for VTE [34,49]. Addressing these critical gaps will be essential to ensure appropriate VTE prevention in patients with cancer, ultimately leading to improved clinical outcomes and patient quality of life.
Authorship Contributions
Conceptualization and writing: NV, and CA. Visualization: NV
Funding
The authors received no funding for this article.
Conflicts of interest
NV has no conflicts of interest to declare. CA has received personal fees for lectures and/or participation in advisory boards from Bayer, Daiichi-Sankyo, BMS/Pfizer alliance and Sanofi.
Correspondence:
Prof. Cihan Ay, MD
Divison of Haematology and Haemostaseology
Department of Medicine I, Medical University of Vienna
Waehringer Guertel 18-20, 1090 Vienna, Austria
T: +43 (0)1 40400-66170
cihan.ay@meduniwien.ac.at
Keywords:
Cancer-associated thrombosis, venous thromboembolism, risk assessment, primary prophylaxis, cancer
References
1. Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TML, Myklebust TÅ, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019 Nov;20(11):1493–505.
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63.
3. Gambardella V, Tarazona N, Cejalvo JM, Lombardi P, Huerta M, Roselló S, et al. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers. 2020 Apr 19;12(4):1009.
4. Emery J, Butow P, Lai-Kwon J, Nekhlyudov L, Rynderman M, Jefford M. Management of common clinical problems experienced by survivors of cancer. Lancet Lond Engl. 2022 Apr 16;399(10334):1537–50.
5. Grilz E, Königsbrügge O, Posch F, Schmidinger M, Pirker R, Lang IM, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018 Sep;103(9):1549–56.
6. Vladic N, Englisch C, Ay C, Pabinger I. Risk assessment and prevention of cancer-associated venous thromboembolism in ambulatory patients with solid malignancies. Res Pract Thromb Haemost. 2024 Dec 24;102664.
7. Amin A, Neuman WR, Lingohr-Smith M, Menges B, Lin J. Venous Thromboembolism Prophylaxis and Risk in the Inpatient and Outpatient Continuum of Care Among Hospitalized Acutely Ill Patients in the US: A Retrospective Analysis. Adv Ther. 2019 Jan 1;36(1):59–71.
8. Girardi L, Wang TF, Ageno W, Carrier M. Updates in the Incidence, Pathogenesis, and Management of Cancer and Venous Thromboembolism. Arterioscler Thromb Vasc Biol. 2023 Jun;43(6):824–31.
9. Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res. 2006;118(5):555–68.
10. Ma Z, Sun X, Zhang Y, Li H, Sun D, An Z, et al. Risk of Thromboembolic Events in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-analysis of Randomized Controlled Trials. Thromb Haemost. 2022 Oct;122(10):1757–66.
11. Das A, Mahapatra S, Bandyopadhyay D, Samanta S, Chakraborty S, Philpotts LL, et al. Bleeding with vascular endothelial growth factor tyrosine kinase inhibitor: A network meta-analysis. Crit Rev Oncol Hematol. 2021 Jan;157:103186.
12. Steiner D, Nopp S, Hoberstorfer T, Pabinger I, Weber B, Ay C. Anxiety in patients with venous thromboembolism: Quantification and risk factors in a prospective cohort study. J Thromb Haemost JTH. 2024 Aug 7;
13. Khorana AA, Mackman N, Falanga A, Pabinger I, Noble S, Ageno W, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primer. 2022 Feb 17;8(1):1–18.
14. Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010 Jun;125(6):490–3.
15. Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021 Feb 11;5(4):927–74.
16. Farge D, Frere C, Connors JM, Khorana AA, Kakkar A, Ay C, et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022 Jul;23(7):e334–47.
17. Falanga A, Ay C, Nisio MD, Gerotziafas G, Jara-Palomares L, Langer F, et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline☆. Ann Oncol. 2023 May 1;34(5):452–67.
18. Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Guideline Update. J Clin Oncol. 2023 Jun;41(16):3063–71.
19. Alikhan R, Gomez K, Maraveyas A, Noble S, Young A, Thomas M, et al. Cancer-associated venous thrombosis in adults (second edition): A British Society for Haematology Guideline. Br J Haematol. 2024;205(1):71–87.
20. Wang T, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018 May 1;2(3):429–38.
21. Nisio MD, Porreca E, Otten HM, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy – Di Nisio, M – 2014 | Cochrane Library. [cited 2025 Apr 11]; Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008500.pub3/full
22. Khorana AA, Francis CW, Kuderer NM, Carrier M, Ortel TL, Wun T, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Thromb Res. 2017 Mar 1;151:89–95.
23. Alexander M, Harris S, Underhill C, Torres J, Sharma S, Lee N, et al. Risk-Directed Ambulatory Thromboprophylaxis in Lung and Gastrointestinal Cancers: The TARGET-TP Randomized Clinical Trial. JAMA Oncol. 2023 Nov 1;9(11):1536–45.
24. Mulder FI, Bosch FTM, van Es N. Primary Thromboprophylaxis in Ambulatory Cancer Patients: Where Do We Stand? Cancers. 2020 Feb 5;12(2):367.
25. Potere N, Barco S, Mahé I, Cesarman‐Maus G, Angchaisuksiri P, Leader A, et al. Awareness of venous thromboembolism among patients with cancer: Preliminary findings from a global initiative for World Thrombosis Day. J Thromb Haemost. 2022 Dec;20(12):2964–71.
26. Englisch C, Moik F, Steiner D, Starzer AM, Berghoff AS, Preusser M, et al. Bleeding events in patients with cancer: incidence, risk factors, and impact on prognosis in a prospective cohort study. Blood. 2024 Aug 27;blood.2024025362.
27. Wang TF, Hill M, Mallick R, Chaudry H, Unachukwu U, Delluc A, et al. The prevalence of relevant drug-drug interactions and associated clinical outcomes in patients with cancer-associated thrombosis on concurrent anticoagulation and anticancer or supportive care therapies. Thromb Res. 2023 Nov;231:128–34.
28. Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012 Aug;97(8):1158–64.
29. Gotta J, Gruenewald LD, Eichler K, Martin SS, Mahmoudi S, Booz C, et al. Unveiling the diagnostic enigma of D-dimer testing in cancer patients: Current evidence and areas of application. Eur J Clin Invest. 2023 Oct;53(10):e14060.
30. Wang Y, Kondrat K, Adhikari J, Nguyen Q, Yu Q, Uprety D. Survival trends among patients with metastatic non-small cell lung cancer before and after the approval of immunotherapy in the United States: A Surveillance, Epidemiology, and End Results database-based study. Cancer. 2025 Jan 1;131(1):e35476.
31. Weiss SA, Wolchok JD, Sznol M. Immunotherapy of Melanoma: Facts and Hopes. Clin Cancer Res Off J Am Assoc Cancer Res. 2019 Sep 1;25(17):5191–201.
32. Prasad V, Haslam A, Olivier T. Updated estimates of eligibility and response: Immune checkpoint inhibitors. J Clin Oncol. 2024 Jun;42(16_suppl):e14613–e14613.
33. Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, et al. Immune checkpoint therapy—current perspectives and future directions. Cell. 2023 Apr 13;186(8):1652–69.
34. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019 Sep;16(9):563–80.
35. Wilhelm G, Mertowska P, Mertowski S, Przysucha A, Strużyna J, Grywalska E, et al. The Crossroads of the Coagulation System and the Immune System: Interactions and Connections. Int J Mol Sci. 2023 Aug 8;24(16):12563.
36. van Lent A, Puscasu R, Kaasjager KAH, Haitjema S, Suelmann BBM, Verhaar MC, et al. Venous and arterial thrombosis in patients receiving immune checkpoint inhibitors. PloS One. 2025;20(4):e0321112.
37. Moik F, Chan WSE, Wiedemann S, Hoeller C, Tuchmann F, Aretin MB, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021 Mar 25;137(12):1669–78.
38. le Sève JD, Guédon AF, Bordenave S, Agard C, Connault J, Pistorius MA, et al. Risk Factors of Venous Thromboembolic Disease in Cancer Patients Treated with Immune Checkpoint Inhibitor. Thromb Haemost. 2023 Nov;123(11):1049–56.
39. Khorana AA, Palaia J, Rosenblatt L, Pisupati R, Huang N, Nguyen C, et al. Venous thromboembolism incidence and risk factors associated with immune checkpoint inhibitors among patients with advanced non-small cell lung cancer. J Immunother Cancer. 2023 Jan 1;11(1):e006072.
40. Lin J, Li W, Zhang X, Zhou K, Yang Y, Cheng S, et al. Thromboembolic events associated with immune checkpoint inhibitors in cancer patients: A Bayesian network meta-analysis. Thromb Res. 2025 Feb;246:109243.
41. McCrae KR, Swaidani S, Diaz-Montero CM, Khorana AA. Old is new again: emergence of thromboembolic complications in cancer patients on immunotherapy. Thromb Res. 2022 May;213(Suppl 1):S51–7.
42. Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation. 2020 Dec 15;142(24):2299–311.
43. Zhu J, Chen Y, Zhang Y, Wang W, Wang Y, Lu Z, et al. Association of immune checkpoint inhibitors therapy with arterial thromboembolic events in cancer patients: A retrospective cohort study. Cancer Med. 2023 Aug 16;12(18):18531–41.
44. Naqash AR, Moey MYY, Cherie Tan XW, Laharwal M, Hill V, Moka N, et al. Major Adverse Cardiac Events With Immune Checkpoint Inhibitors: A Pooled Analysis of Trials Sponsored by the National Cancer Institute-Cancer Therapy Evaluation Program. J Clin Oncol Off J Am Soc Clin Oncol. 2022 Oct 10;40(29):3439–52.
45. Drobni ZD, Gongora C, Taron J, Suero-Abreu GA, Karady J, Gilman HK, et al. Impact of immune checkpoint inhibitors on atherosclerosis progression in patients with lung cancer. J Immunother Cancer. 2023 Jul 1;11(7):e007307.
46. Lou L, Detering L, Luehmann H, Amrute JM, Sultan D, Ma P, et al. Visualizing Immune Checkpoint Inhibitors Derived Inflammation in Atherosclerosis. Circ Res. 2024 Oct 25;135(10):990–1003.
47. Zhao ML, Liang C, Jiang WW, Zhang M, Guan H, Hong Z, et al. Inhibition of CTLA-4 accelerates atherosclerosis in hyperlipidemic mice by modulating the Th1/Th2 balance via the NF-κB signaling pathway. Heliyon. 2024 Sep 15;10(17):e37278.
48. Vladić N, Englisch C, Berger JM, Moik F, Berghoff AS, Preusser M, et al. Validation of Risk Assessment Models for Venous Thromboembolism in Cancer Patients Receiving Systemic Therapies. Blood Adv. 2025 Mar 21;bloodadvances.2025016044.
49. Moik F, Absenger G, Wurm R, Hochmair MJ, Ay C. Prevention and Treatment of Venous Thromboembolism Associated with Amivantamab-Based Therapies in Patients with Lung Cancer—Provisional Clinical Opinion Based on Existing Clinical Practice Guidelines. Cancers. 2025 Jan;17(2):259.
50. Icht O, Darzi N, Shimony S, Jacobi O, Reinhorn D, Landman Y, et al. Venous thromboembolism incidence and risk assessment in lung cancer patients treated with immune checkpoint inhibitors. J Thromb Haemost JTH. 2021 May;19(5):1250–8.
51. Muñoz-Unceta N, Zugazagoitia J, Manzano A, Jiménez-Aguilar E, Olmedo ME, Cacho JD, et al. High risk of thrombosis in patients with advanced lung cancer harboring rearrangements in ROS1. Eur J Cancer Oxf Engl 1990. 2020 Dec;141:193–8.
52. Liu Y, Wang W, Wu F, Gao G, Xu J, Li X, et al. High discrepancy in thrombotic events in non-small cell lung cancer patients with different genomic alterations. Transl Lung Cancer Res. 2021 Mar;10(3):1512–24.
53. Hao L, Zhang J, Di Y, Qi Z, Zhang P. Predicting a failure of postoperative thromboprophylaxis in non-small cell lung cancer: A stacking machine learning approach. PLOS ONE. 2025 Apr 1;20(4):e0320674.
54. Ording AG, Christensen TD, Skjøth F, Noble S, Højen AA, Mørkved AL, et al. Risk of Venous Thromboembolism in Patients With Stage III and IV Non–Small-Cell Lung Cancer: Nationwide Descriptive Cohort Study. Clin Lung Cancer. 2024 Jul 1;25(5):407-416.e1.
55. Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012 Jun;7(3):291–2.
56. Moik F, van Es N, Posch F, Di Nisio M, Fuereder T, Preusser M, et al. Gemcitabine and Platinum-Based Agents for the Prediction of Cancer-Associated Venous Thromboembolism: Results from the Vienna Cancer and Thrombosis Study. Cancers. 2020 Sep 3;12(9):2493.
57. Qi Y, Wang X, Guo T, You T, Wang P. Correlation between ALK+ non-small cell lung cancer targeted therapy and thrombosis: a systematic review and network meta-analysis. BMJ Open. 2024 Sep 30;14(9):e078173.
58. Moik F, Riedl JM, Ay C. VTE with Amivantamab plus Chemotherapy in NSCLC. N Engl J Med. 2024 Feb 7;390(6):578–80.
