Patients with cancer are at an increased risk of venous and arterial thromboembolism (VTE/ATE) [1, 2]. A number of factors have been identified that contribute to this risk [3, 4]. Among them, treatment-related risk factors, such as platinum-based chemotherapy, anti-angiogenic agents and hormone-based therapy, have been associated with an increase in risk of thrombotic events in patients with cancer [3, 5-8].
Immune checkpoint inhibitors are increasingly used for the treatment of different cancers. By targeting the tumours’ immune escape mechanisms, these agents have led to a substantially improved prognosis in patients with melanoma, non-small-cell lung cancer, renal cell carcinoma and other cancers. Large-scale randomized controlled trials evaluating immune checkpoint inhibitors surprisingly did not provide information on rates of VTE and/or ATE as adverse events [9]. Currently, only limited data on risk of VTE associated with immune checkpoint inhibitors are available. Small retrospective cohort studies have reported rates of VTE between 6% and 18%, and in several case reports dramatic and fatal thromboembolic events (both VTE and ATE) during immune checkpoint inhibitor therapy were described [10-14].
To provide more evidence, a single center retrospective cohort study including consecutive patients treated with an immune checkpoint inhibitor at the Medical University of Vienna, Austria, between 2015 and 2018 was initiated [15]. The aim was to investigate the frequency, potential risk factors, and clinical consequences of VTE and ATE in a large and unselected single-institutional cohort.
In total, 672 patients treated with at least one cycle of either nivolumab (42%), pembrolizumab (40%), ipilimumab (7%), ipilimumab + nivolumab (6%), atezolizumab (5%), or avelumab (1%) were included. Median age of patients was 64 years (interquartile range, 54–72); 39% were female. The most frequent cancer diagnoses were melanoma (30%), non-small-cell lung cancer (24%), renal-cell carcinoma (11%), head and neck cancer (10%) and urothelial cancer (5%), and most patients had distant metastasis at therapy initiation (86%).
Risk of venous and arterial thromboembolism
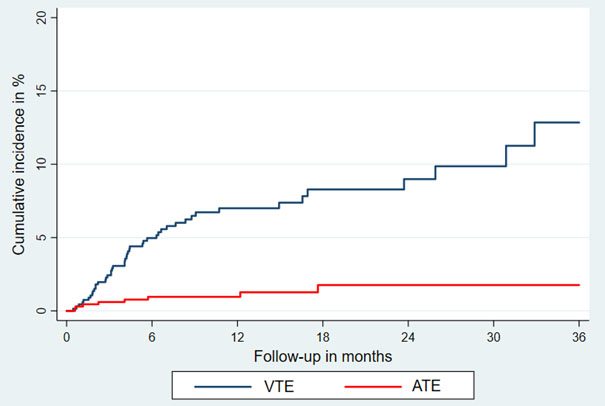
Patients were followed from the first day of initiation of immune checkpoint inhibitor therapy until death, any subsequent medical anti-cancer therapy (e.g., chemotherapy), or for 3 months after the last therapy cycle (median follow-up: 8.5 months [interquartile range: 7.6–9.6]). During this time, 47 VTE (18 pulmonary embolism [PE], 17 deep vein thrombosis [DVT], three PE+DVT, four catheter-related thrombosis, five visceral vein thrombosis), and nine ATE (four myocardial infarctions, three ischemic strokes, two acute peripheral arterial occlusions) were observed. Of those, two fatal PE and one fatal ATE (ischemic stroke) were recorded. The cumulative incidence in competing risk analysis, accounting for all-cause mortality as competing outcome event, was 12.9 % for VTE (95% CI: 8.2–18.5) and 1.8% for ATE (95% CI: 0.7–3.6).
Cumulative incidence of venous and arterial thromboembolism during immune checkpoint inhibitor therapy in competing risk analysis.
Clinical consequences of thrombotic events
The occurrence of VTE was associated with increased mortality in a multi-state model (transition hazard ratio [THR] for death: 3.09 [95% CI: 2.07–4.60]), with median overall survival estimates after VTE of only 11.6 months compared with 25.5 months without VTE. In addition, VTE seems to be indicative of treatment failure, as the occurrence of VTE was associated with a markedly decreased progression-free survival (THR for disease progression: 3.63 [95% CI: 2.47–5.36]). Furthermore, the occurrence of VTE led to delay in immune checkpoint inhibitor therapy in 11% of VTE patients for a median of 7 days, and ATE led to treatment discontinuation in one patient and a substantial delay of between 2 and 5 months in three patients.
After the index VTE, anticoagulation treatment was frequently complicated by recurrent VTE (9%) and bleeding events, including major bleeding in 4% and clinically relevant non-major bleeding in 9%. These findings highlight the unfavourable clinical impact of venous and arterial thromboembolism on the clinical course of patients treated with immune checkpoint inhibitors.
Risk factors and subgroup analysis
The strongest identified risk factor for VTE was a positive history of VTE (subdistribution HR [SHR]: 3.69 [95% CI: 2.00–6.81]), and the presence of distant metastasis was non-significantly associated with VTE risk (subdistribution HR: 1.71, 95% CI: 0.62–4.73). Interestingly, no association with VTE was observed for ECOG performance status, the Charlson Comorbidity Index, sex, age, BMI, grade, or the Khorana score. Further, rates of VTE were highly comparable between subgroups of tumour types and immune checkpoint inhibitor agents.
Conclusions
In conclusion, substantial rates of VTE and ATE were found in this large, unselected single-institutional cohort study [15]. Whether these rates are causally related to the treatment with immune checkpoint inhibitors itself or rather represent the underlying thrombotic risk profile of included patients cannot be answered. However, irrespective of potential causality, the identification of secondary causes of morbidity and mortality such as VTE and ATE under this novel and highly effective anti-cancer treatment, which can potentially lead to long term remission and survival in patients with advanced cancer, is of utmost importance. Future prospective studies are needed to better understand the risk of VTE and ATE associated with immune checkpoint inhibitors and to identify risk factors for predicting VTE and ATE to improve patient care by preventing thromboembolic complications.
References
- Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost. 2017;117(1):57-65.
- Grilz E, Königsbrügge O, Posch F, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103(9):1549-1556.
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219-230.
- Moik F, Ay C, Pabinger I. Risk prediction for cancer-associated thrombosis in ambulatory patients with cancer: past, present and future. Thrombosis Res. 2020;191:S3-S11.
- Seng S, Liu Z, Chiu SK, et al. Risk of venous thromboembolism in patients with cancer treated with Cisplatin: a systematic review and meta-analysis. J Clin Oncol. 2012;30(35):4416-4426.
- Proverbs-Singh T, Chiu SK, Liu Z, et al. Arterial thromboembolism in cancer patients treated with cisplatin: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104(23):1837-1840.
- Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300(19):2277-2285.
- Totzeck M, Mincu RI, Rassaf T. Cardiovascular Adverse Events in Patients With Cancer Treated With Bevacizumab: A Meta-Analysis of More Than 20 000 Patients. J Am Heart Assoc. 2017;6(8):e006278. Published 2017 Aug 10.
- Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008-1019.
- Ibrahimi S, Machiorlatti M, Vesely SK, et al. Incidence of vascular thromboembolic events in patients receiving immunotherapy: a single institution experience. Blood. 2017;130(suppl 1):4864.
- Hegde AM, Stroud CRG, Cherry CR, Yogarajah M, Cherukuri SD, Walker PR. Incidence and impact of thromboembolic events in lung cancer patients treated with nivolumab. J Clin Oncol. 2017;35(15_suppl):e20624-e20624.
- Boutros C, Scoazec J-Y, Mateus C, Routier E, Roy S, Robert C. Arterial thrombosis and anti-PD-1 blockade. Eur J Cancer 2018;91:164-166.
- Ando Y, Hayashi T, Sugimoto R, et al. Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs. 2020;38(4):1200-1206
- Nichetti F, Ligorio F, Zattarin E, et al. Is There an Interplay between Immune Checkpoint Inhibitors, Thromboprophylactic Treatments and Thromboembolic Events? Mechanisms and Impact in Non-Small Cell Lung Cancer Patients. Cancers (Basel). 2019;12(1):67. Published 2019 Dec 25.
- Moik F, Chan WE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137(12):1669-1678.
